当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The nature of active centers catalyzing oxygen electro-reduction at platinum surfaces in alkaline media†
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2018-12-04 00:00:00 , DOI: 10.1039/c8ee03228a Yunchang Liang 1, 2, 3, 4 , David McLaughlin 1, 2, 3, 4 , Christoph Csoklich 1, 2, 3, 4 , Oliver Schneider 2, 4, 5, 6 , Aliaksandr S. Bandarenka 1, 2, 3, 4, 7
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2018-12-04 00:00:00 , DOI: 10.1039/c8ee03228a Yunchang Liang 1, 2, 3, 4 , David McLaughlin 1, 2, 3, 4 , Christoph Csoklich 1, 2, 3, 4 , Oliver Schneider 2, 4, 5, 6 , Aliaksandr S. Bandarenka 1, 2, 3, 4, 7
Affiliation
|
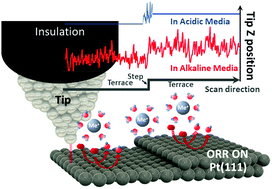
Energy conversion devices that use OH-conducting electrolytes are becoming more and more attractive with recent substantial progress in the development and commercialization of relevant membrane materials and systems. However, the activities of numerous catalysts towards oxygen electro-reduction taking place at the cathodes of such devices in alkaline media are surprisingly different from those in acidic electrolytes. This is for instance the case for Pt and Pt-alloy electrodes, which demonstrate unexpected and drastic variations in activity depending on the electrolyte pH and surface structure. Here we apply recently introduced electrochemical scanning tunnelling microscopy noise measurements to directly identify active centers at Pt(111)-based surfaces in three alkaline electrolytes (LiOH, KOH and CsOH) under reaction conditions. For all three solutions it was found that the most active sites are located on the Pt(111) terraces, in contrast to the acidic media, where concave defects significantly increase the oxygen reduction activity. These defect-centers are to all practical purposes deactivated in alkaline media. This not only explains the above-mentioned activity differences between acidic and alkaline electrolytes but also suggests strategies to design nanostructured Pt-electrocatalysts for applications at high pH values.
中文翻译:

碱性介质中活性中心在铂表面催化氧还原的性质†
随着相关膜材料和系统的开发和商业化的最新实质性进展,使用含OH的电解质的能量转换装置变得越来越有吸引力。但是,令人惊讶的是,在碱性介质中在这种装置的阴极处发生的许多催化剂对氧电还原的活性与酸性电解质中的那些令人惊奇地不同。例如,Pt和Pt合金电极就是这种情况,它们根据电解液的pH值和表面结构表现出出乎意料的剧烈变化。在这里,我们应用了最近引入的电化学扫描隧道显微镜噪声测量,以在反应条件下直接识别三种碱性电解质(LiOH,KOH和CsOH)中基于Pt(111)的表面上的活性中心。对于所有三种解决方案,发现与酸性介质相比,活性最强的位点位于Pt(111)平台上,在酸性介质中,凹形缺陷会显着增加氧还原活性。这些缺陷中心实际上在碱性介质中已失效。这不仅解释了酸性和碱性电解质之间的上述活性差异,而且提出了设计用于高pH值应用的纳米结构Pt电催化剂的策略。
更新日期:2018-12-04
中文翻译:

碱性介质中活性中心在铂表面催化氧还原的性质†
随着相关膜材料和系统的开发和商业化的最新实质性进展,使用含OH的电解质的能量转换装置变得越来越有吸引力。但是,令人惊讶的是,在碱性介质中在这种装置的阴极处发生的许多催化剂对氧电还原的活性与酸性电解质中的那些令人惊奇地不同。例如,Pt和Pt合金电极就是这种情况,它们根据电解液的pH值和表面结构表现出出乎意料的剧烈变化。在这里,我们应用了最近引入的电化学扫描隧道显微镜噪声测量,以在反应条件下直接识别三种碱性电解质(LiOH,KOH和CsOH)中基于Pt(111)的表面上的活性中心。对于所有三种解决方案,发现与酸性介质相比,活性最强的位点位于Pt(111)平台上,在酸性介质中,凹形缺陷会显着增加氧还原活性。这些缺陷中心实际上在碱性介质中已失效。这不仅解释了酸性和碱性电解质之间的上述活性差异,而且提出了设计用于高pH值应用的纳米结构Pt电催化剂的策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号