当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Peptide-Membrane Interactions Affect the Inhibitory Potency and Selectivity of Spider Toxins ProTx-II and GpTx-1.
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-12-21 , DOI: 10.1021/acschembio.8b00989 Nicole Lawrence 1 , Bin Wu , Joseph Ligutti 1 , Olivier Cheneval 1 , Akello Joanna Agwa 1 , Aurélie H Benfield 1 , Kaustav Biswas , David J Craik , Les P Miranda , Sónia Troeira Henriques 1 , Christina I Schroeder 1
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-12-21 , DOI: 10.1021/acschembio.8b00989 Nicole Lawrence 1 , Bin Wu , Joseph Ligutti 1 , Olivier Cheneval 1 , Akello Joanna Agwa 1 , Aurélie H Benfield 1 , Kaustav Biswas , David J Craik , Les P Miranda , Sónia Troeira Henriques 1 , Christina I Schroeder 1
Affiliation
|
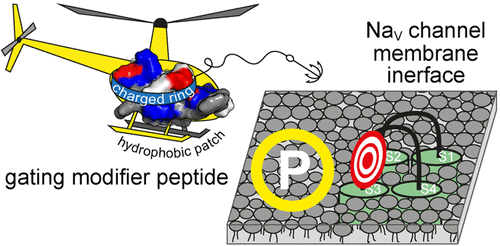
Gating modifier toxins (GMTs) from spider venom can inhibit voltage gated sodium channels (NaVs) involved in pain signal transmission, including the NaV1.7 subtype. GMTs have a conserved amphipathic structure that allow them to interact with membranes and also with charged residues in regions of NaV that are exposed at the cell surface. ProTx-II and GpTx-1 are GMTs able to inhibit NaV1.7 with high potency, but they differ in their ability to bind to membranes and in their selectivity over other NaV subtypes. To explore these differences and gain detailed information on their membrane-binding ability and how this relates to potency and selectivity, we examined previously described NaV1.7 potent/selective GpTx-1 analogues and new ProTx-II analogues designed to reduce membrane binding and improve selectivity for NaV1.7. Our studies reveal that the number and type of hydrophobic residues as well as how they are presented at the surface determine the affinity of ProTx-II and GpTx-1 for membranes and that altering these residues can have dramatic effects on NaV inhibitory activity. We demonstrate that strong peptide-membrane interactions are not essential for inhibiting NaV1.7 and propose that hydrophobic interactions instead play an important role in positioning the GMT at the membrane surface proximal to exposed NaV residues, thereby affecting peptide-channel interactions. Our detailed structure-activity relationship study highlights the challenges of designing GMT-based molecules that simultaneously achieve high potency and selectivity for NaV1.7, as single mutations can induce local changes in GMT structure that can have a major impact on NaV-inhibitory activity.
中文翻译:

肽膜相互作用影响蜘蛛毒素ProTx-II和GpTx-1的抑制能力和选择性。
来自蜘蛛毒的门控修饰物毒素(GMT)可以抑制与疼痛信号传递有关的电压门控钠通道(NaV),包括NaV1.7亚型。GMT具有保守的两亲结构,使它们能够与膜以及在细胞表面暴露的NaV区域中的带电荷残基相互作用。ProTx-II和GpTx-1是能够高效抑制NaV1.7的GMT,但它们结合膜的能力和相对于其他NaV亚型的选择性不同。为了探索这些差异并获得有关它们的膜结合能力以及与效力和选择性之间的关系的详细信息,我们检查了先前描述的NaV1.7强效/选择性GpTx-1类似物和旨在减少膜结合和改善的新ProTx-II类似物。 NaV1.7的选择性。我们的研究表明,疏水残基的数量和类型以及它们在表面上的呈现方式决定了ProTx-II和GpTx-1对膜的亲和力,改变这些残基对NaV抑制活性可能具有显着影响。我们证明强肽-膜相互作用对于抑制NaV1.7并不是必不可少的,并建议疏水性相互作用反而在将GMT定位在暴露于NaV残基的近膜表面上起重要作用,从而影响肽-通道相互作用。我们详细的结构-活性关系研究强调了设计基于GMT的分子所面临的挑战,这些分子同时实现了NaV1.7的高效性和选择性,
更新日期:2018-12-03
中文翻译:

肽膜相互作用影响蜘蛛毒素ProTx-II和GpTx-1的抑制能力和选择性。
来自蜘蛛毒的门控修饰物毒素(GMT)可以抑制与疼痛信号传递有关的电压门控钠通道(NaV),包括NaV1.7亚型。GMT具有保守的两亲结构,使它们能够与膜以及在细胞表面暴露的NaV区域中的带电荷残基相互作用。ProTx-II和GpTx-1是能够高效抑制NaV1.7的GMT,但它们结合膜的能力和相对于其他NaV亚型的选择性不同。为了探索这些差异并获得有关它们的膜结合能力以及与效力和选择性之间的关系的详细信息,我们检查了先前描述的NaV1.7强效/选择性GpTx-1类似物和旨在减少膜结合和改善的新ProTx-II类似物。 NaV1.7的选择性。我们的研究表明,疏水残基的数量和类型以及它们在表面上的呈现方式决定了ProTx-II和GpTx-1对膜的亲和力,改变这些残基对NaV抑制活性可能具有显着影响。我们证明强肽-膜相互作用对于抑制NaV1.7并不是必不可少的,并建议疏水性相互作用反而在将GMT定位在暴露于NaV残基的近膜表面上起重要作用,从而影响肽-通道相互作用。我们详细的结构-活性关系研究强调了设计基于GMT的分子所面临的挑战,这些分子同时实现了NaV1.7的高效性和选择性,

























 京公网安备 11010802027423号
京公网安备 11010802027423号