npj Precision Oncology ( IF 7.9 ) Pub Date : 2018-12-04 , DOI: 10.1038/s41698-018-0070-1 Malak Abedalthagafi , Duna Barakeh , Kara M. Foshay
|
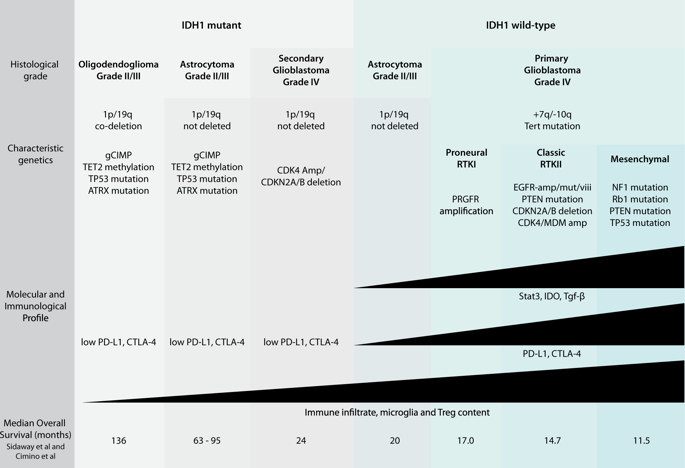
The prognosis of glioblastoma has changed little over the past two decades, with only minor improvements in length of overall survival through the addition of temozolomide (temodal) to standard of care and the recommended use of alternating electric field therapy (optune) to newly diagnosed patients. In an effort to define novel therapeutic targets across molecularly heterogeneous disease subgroups, researchers have begun to uncover the complex interplay between epigenetics, cell signaling, metabolism, and the immunosuppressive tumor microenvironment. Indeed, IDH mutations are now recognized as a defining differential factor not only influencing global hypermethylation and patient prognosis but also degree of immune infiltration within individual tumors. Likewise, next-generation sequencing has defined subgroup-specific transcriptional profiles that correlate with different mechanisms of immune evasion, including increased PD-L1 and CTLA-4 among mesenchymal tumors. Interestingly, sequencing of the T cell repertoire from numerous patient samples suggests that the correlation between mutational burden and enrichment of tumor-specific peptides may be less convincing than originally suspected. While this raises questions over the efficacy of dendritic cell or tumor-lysate vaccines and CAR-T therapies, these avenues continue to be explored. In addition to these active immunotherapies, inhibitors of molecular hubs with wide reaching effects, including STAT3, IDO, and TGF-β, are now in early-phase clinical trials. With the potential to block intrinsic biological properties of tumor growth and invasion while bolstering the immunogenic profile of the tumor microenvironment, these new targets represent a new direction for GBM therapies. In this review, we show the advances in molecular profiling and immunophenotyping of GBM, which may lead to the development of new personalized therapeutic strategies.
中文翻译:

胶质母细胞瘤的免疫遗传学:个性化患者管理的未来
在过去的二十年中,胶质母细胞瘤的预后变化不大,通过在标准治疗中添加替莫唑胺(temodal)和建议对新诊断的患者推荐使用交替电场治疗(optune),总生存期仅略有改善。为了在分子异质性疾病亚组之间定义新的治疗靶标,研究人员开始发现表观遗传学,细胞信号传导,新陈代谢和免疫抑制性肿瘤微环境之间的复杂相互作用。实际上,IDH突变现已被认为是决定性的差异因素,不仅影响整体甲基化程度高和患者的预后,而且影响单个肿瘤内的免疫浸润程度。同样地,下一代测序已定义了与免疫逃逸的不同机制相关的亚组特异性转录谱,包括间充质肿瘤中PD-L1和CTLA-4的增加。有趣的是,对来自众多患者样品的T细胞库的测序表明,突变负荷与肿瘤特异性肽富集之间的相关性可能比最初怀疑的要少。尽管这引起了对树突状细胞或肿瘤溶解产物疫苗和CAR-T疗法功效的质疑,但仍在探索这些途径。除了这些主动免疫疗法外,具有广泛影响的分子集线器抑制剂,包括STAT3,IDO和TGF-β,目前正在早期临床试验中。这些新靶标具有阻断肿瘤生长和侵袭的内在生物学特性,同时增强肿瘤微环境的免疫原性的潜力,代表了GBM治疗的新方向。在这篇综述中,我们显示了GBM的分子谱分析和免疫表型研究的进展,这可能会导致新的个性化治疗策略的发展。


























 京公网安备 11010802027423号
京公网安备 11010802027423号