当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Monoamine Biosynthesis via a Noncanonical Calcium-Activatable Aromatic Amino Acid Decarboxylase in Psilocybin Mushroom.
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-12-10 , DOI: 10.1021/acschembio.8b00821 Michael Patrick Torrens-Spence 1 , Chun-Ting Liu 1, 2, 3 , Tomáš Pluskal 1 , Yin Kwan Chung 1, 4 , Jing-Ke Weng 1, 2
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-12-10 , DOI: 10.1021/acschembio.8b00821 Michael Patrick Torrens-Spence 1 , Chun-Ting Liu 1, 2, 3 , Tomáš Pluskal 1 , Yin Kwan Chung 1, 4 , Jing-Ke Weng 1, 2
Affiliation
|
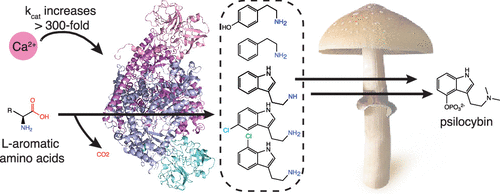
Aromatic l-amino acid decarboxylases (AAADs) are a phylogenetically diverse group of enzymes responsible for the decarboxylation of aromatic amino acid substrates into their corresponding aromatic arylalkylamines. AAADs have been extensively studied in mammals and plants as they catalyze the first step in the production of neurotransmitters and bioactive phytochemicals, respectively. Unlike mammals and plants, the hallucinogenic psilocybin mushroom Psilocybe cubensis reportedly employs an unrelated phosphatidylserine-decarboxylase-like enzyme to catalyze l-tryptophan decarboxylation, the first step in psilocybin biosynthesis. To explore the origin of this chemistry in psilocybin mushroom, we generated the first de novo transcriptomes of P. cubensis and investigated several putative l-tryptophan-decarboxylase-like enzymes. We report the biochemical characterization of a noncanonical AAAD from P. cubensis ( PcncAAAD) that exhibits substrate permissiveness toward l-phenylalanine, l-tyrosine, and l-tryptophan, as well as chloro-tryptophan derivatives. The crystal structure of PcncAAAD revealed the presence of a unique C-terminal appendage domain featuring a novel double-β-barrel fold. This domain is required for PcncAAAD activity and regulates catalytic rate and thermal stability through calcium binding. PcncAAAD likely plays a role in psilocybin production in P. cubensis and offers a new tool for metabolic engineering of aromatic-amino-acid-derived natural products.
中文翻译:

介壳菌蘑菇中通过非规范性钙激活芳香族氨基酸脱羧酶的单胺生物合成。
芳族1-氨基酸脱羧酶(AAAD)是系统上多样化的一组酶,其负责使芳族氨基酸底物脱羧成其相应的芳族芳基烷基胺。由于AAAD分别催化神经递质和生物活性植物化学物质生产的第一步,因此已在哺乳动物和植物中进行了广泛的研究。据报道,与哺乳动物和植物不同,致幻性鹦鹉螺菌蘑菇Psilocybe cubensis使用一种不相关的磷脂酰丝氨酸-脱羧酶样酶催化色氨酸脱羧,这是鹦鹉螺菌素生物合成的第一步。为了探索这种化学在鹦鹉螺菌属蘑菇中的起源,我们产生了立方体假单胞菌的第一个从头转录组,并研究了几种推定的L-色氨酸-脱羧酶样酶。我们报告了非典型的AAAD的生化特征,从立方假单胞菌(PcncAAAD)表现出对L-苯丙氨酸,L-酪氨酸和L-色氨酸以及氯-色氨酸衍生物的底物允许性。PcncAAAD的晶体结构揭示了一个独特的C端附件结构域的存在,其特征是新颖的双β-桶状折叠。该结构域是PcncAAAD活性所必需的,并通过钙结合调节催化速率和热稳定性。PcncAAAD可能在立方体假单胞菌的psilocybin生产中发挥作用,并为芳香族氨基酸衍生的天然产物的代谢工程提供了新的工具。PcncAAAD的晶体结构揭示了一个独特的C端附件结构域的存在,其特征是新颖的双β-桶状折叠。该结构域是PcncAAAD活性所必需的,并通过钙结合调节催化速率和热稳定性。PcncAAAD可能在立方体假单胞菌的psilocybin生产中发挥作用,并为芳香族氨基酸衍生的天然产物的代谢工程提供了新的工具。PcncAAAD的晶体结构揭示了一个独特的C端附件结构域的存在,其特征是新颖的双β-桶状折叠。该结构域是PcncAAAD活性所必需的,并通过钙结合调节催化速率和热稳定性。PcncAAAD可能在立方体假单胞菌的psilocybin生产中发挥作用,并为芳香族氨基酸衍生的天然产物的代谢工程提供了新的工具。
更新日期:2018-11-28
中文翻译:

介壳菌蘑菇中通过非规范性钙激活芳香族氨基酸脱羧酶的单胺生物合成。
芳族1-氨基酸脱羧酶(AAAD)是系统上多样化的一组酶,其负责使芳族氨基酸底物脱羧成其相应的芳族芳基烷基胺。由于AAAD分别催化神经递质和生物活性植物化学物质生产的第一步,因此已在哺乳动物和植物中进行了广泛的研究。据报道,与哺乳动物和植物不同,致幻性鹦鹉螺菌蘑菇Psilocybe cubensis使用一种不相关的磷脂酰丝氨酸-脱羧酶样酶催化色氨酸脱羧,这是鹦鹉螺菌素生物合成的第一步。为了探索这种化学在鹦鹉螺菌属蘑菇中的起源,我们产生了立方体假单胞菌的第一个从头转录组,并研究了几种推定的L-色氨酸-脱羧酶样酶。我们报告了非典型的AAAD的生化特征,从立方假单胞菌(PcncAAAD)表现出对L-苯丙氨酸,L-酪氨酸和L-色氨酸以及氯-色氨酸衍生物的底物允许性。PcncAAAD的晶体结构揭示了一个独特的C端附件结构域的存在,其特征是新颖的双β-桶状折叠。该结构域是PcncAAAD活性所必需的,并通过钙结合调节催化速率和热稳定性。PcncAAAD可能在立方体假单胞菌的psilocybin生产中发挥作用,并为芳香族氨基酸衍生的天然产物的代谢工程提供了新的工具。PcncAAAD的晶体结构揭示了一个独特的C端附件结构域的存在,其特征是新颖的双β-桶状折叠。该结构域是PcncAAAD活性所必需的,并通过钙结合调节催化速率和热稳定性。PcncAAAD可能在立方体假单胞菌的psilocybin生产中发挥作用,并为芳香族氨基酸衍生的天然产物的代谢工程提供了新的工具。PcncAAAD的晶体结构揭示了一个独特的C端附件结构域的存在,其特征是新颖的双β-桶状折叠。该结构域是PcncAAAD活性所必需的,并通过钙结合调节催化速率和热稳定性。PcncAAAD可能在立方体假单胞菌的psilocybin生产中发挥作用,并为芳香族氨基酸衍生的天然产物的代谢工程提供了新的工具。


























 京公网安备 11010802027423号
京公网安备 11010802027423号