当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
In Vivo Stability Profiles of Anti-factor D Molecules Support Long-Acting Delivery Approaches
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2018-11-16 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00871 Kelly M. Loyet 1 , Philip E. Hass 2 , Wendy N. Sandoval 3 , Ashley Morando 1 , Peter Liu 3 , Whitney Shatz 2 , Leslie Dickmann 4 , Margaret Kenrick 4 , Jeremy Good 5 , Teresa Davancaze 5 , Alyssa M. Morimoto 5 , Robert F. Kelley 6 , Justin M. Scheer 2
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2018-11-16 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00871 Kelly M. Loyet 1 , Philip E. Hass 2 , Wendy N. Sandoval 3 , Ashley Morando 1 , Peter Liu 3 , Whitney Shatz 2 , Leslie Dickmann 4 , Margaret Kenrick 4 , Jeremy Good 5 , Teresa Davancaze 5 , Alyssa M. Morimoto 5 , Robert F. Kelley 6 , Justin M. Scheer 2
Affiliation
|
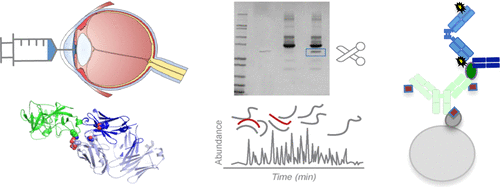
The collection of aqueous humor (phase 1b/2 Mahalo study) from patients dosed intravitreally with anti-factor D (AFD; FCFD4514S, lampalizumab), a humanized antibody fragment previously under investigation to treat geographic atrophy (GA) secondary to age-related macular degeneration, presented a unique opportunity to examine AFD properties in clinical samples. We investigated AFD stability and target-binding characteristics to set up strategies for engineering and evaluating optimized molecules that enable less frequent dosing. Two variants, AFD.v8 and AFD.v14, were evaluated as alternatives to AFD for longer-acting treatments. Mass spectrometry, surface plasmon resonance, and immunoassay were used to assess AFD stability and binding activity in aqueous humor samples from Mahalo patients. In vitro stability and binding activity of AFD, AFD.v8, and AFD.v14 were assessed in human vitreous humor versus buffer at 37 °C over 16 weeks and in vivo in rabbits over 28 days along with pharmacokinetic determinations. In human aqueous humor, AFD specific binding was >85% through 30 days, and deamidation was <3% through 60 days, consistent with the AFD stability and binding activity in vitreous humor from humans in vitro and rabbits in vivo. Target binding, stability, and rabbit pharmacokinetic parameters of AFD.v8 and AFD.v14 were similar to those of AFD. Physiological stability and activity of AFD translated across in vitro and in vivo studies in humans and rabbits. The two variants AFD.v8 and AFD.v14 demonstrated comparable potency and pharmacokinetics. These findings, along with previously demonstrated improved solubility of AFD.v8 and AFD.v14, provide proof-of-concept for developing other similar long-acting therapeutic variants.
中文翻译:

抗因子D分子的体内稳定性概况支持长效递送方法
房水的收集(1b阶段/ 2 Mahalo研究)来自玻璃体腔注射抗因子D(AFD; FCFD4514S,lampalizumab)的患者,这是一种先前正在研究以治疗因年龄相关性黄斑变性而继发的地理萎缩(GA)的人源化抗体片段,临床样品中的AFD特性。我们研究了AFD的稳定性和靶标结合特性,以建立工程设计和评估策略,从而优化了可以减少频繁给药的分子。评估了两个变体AFD.v8和AFD.v14作为长效治疗的AFD替代品。质谱,表面等离振子共振和免疫测定法用于评估Mahalo患者房水样本中的AFD稳定性和结合活性。AFD,AFD.v8和AFD的体外稳定性和结合活性。在37°C下于人玻璃体液中相对于缓冲液评估v14超过16周,而在兔体内在28天中评估v14以及药代动力学测定。在人房水中,AFD特异性结合在30天之内> 85%,脱酰胺化<3%至60天,与人体外和体内兔玻璃体液中AFD的稳定性和结合活性一致。AFD.v8和AFD.v14的靶标结合,稳定性和兔药代动力学参数与AFD相似。AFD的生理稳定性和活性在人和兔子的体内和体外研究中得到了转化。两种变体AFD.v8和AFD.v14表现出可比的功效和药代动力学。这些发现以及先前证明的AFD.v8和AFD.v14的溶解度有所提高,
更新日期:2018-11-16
中文翻译:

抗因子D分子的体内稳定性概况支持长效递送方法
房水的收集(1b阶段/ 2 Mahalo研究)来自玻璃体腔注射抗因子D(AFD; FCFD4514S,lampalizumab)的患者,这是一种先前正在研究以治疗因年龄相关性黄斑变性而继发的地理萎缩(GA)的人源化抗体片段,临床样品中的AFD特性。我们研究了AFD的稳定性和靶标结合特性,以建立工程设计和评估策略,从而优化了可以减少频繁给药的分子。评估了两个变体AFD.v8和AFD.v14作为长效治疗的AFD替代品。质谱,表面等离振子共振和免疫测定法用于评估Mahalo患者房水样本中的AFD稳定性和结合活性。AFD,AFD.v8和AFD的体外稳定性和结合活性。在37°C下于人玻璃体液中相对于缓冲液评估v14超过16周,而在兔体内在28天中评估v14以及药代动力学测定。在人房水中,AFD特异性结合在30天之内> 85%,脱酰胺化<3%至60天,与人体外和体内兔玻璃体液中AFD的稳定性和结合活性一致。AFD.v8和AFD.v14的靶标结合,稳定性和兔药代动力学参数与AFD相似。AFD的生理稳定性和活性在人和兔子的体内和体外研究中得到了转化。两种变体AFD.v8和AFD.v14表现出可比的功效和药代动力学。这些发现以及先前证明的AFD.v8和AFD.v14的溶解度有所提高,


























 京公网安备 11010802027423号
京公网安备 11010802027423号