NeuroToxicology ( IF 3.4 ) Pub Date : 2018-11-03 , DOI: 10.1016/j.neuro.2018.10.012 Gunnar F. Kwakye , Jessica A. Jiménez , Morgan G. Thomas , Brett A. Kingsley , Matthew McIIvin , Mak A. Saito , Edmund M. Korley
|
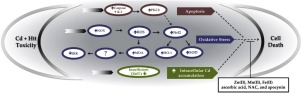
Huntington’s disease (HD) is functionally linked to environmental factors including cigarette use and dyshomeostasis in the levels of metals. Interestingly, one of the most abundant heavy metals in cigarettes is cadmium (Cd), which also accumulates in the striatum and causes neurotoxicity upon exposure. Thus, we hypothesized that heterozygous huntingtin (HTT), responsible for the majority of cases of HD in patients, in combination with Cd exposure would cause neurotoxicity and neurodegeneration via increased intracellular accumulation of Cd and activation of oxidative stress signaling mechanisms in a mouse striatal cell line model of HD. We report that heterozygous HTT striatal cells are significantly more susceptible to Cd-induced cytotoxicity as compared to wild-type HTT cells upon exposure for 48 h.
The heterozygous HTT and Cd-induced cytotoxicity led to a NADPH oxidase (NOX) mediated oxidative stress that was attenuated by exogenous antioxidants and a NOX inhibitor, apocynin. Heterozygous HTT coupled with Cd exposure caused increased expression of protein kinase C δ (PKCδ) and other key oxidative stress proteins levels, enhanced the activation of caspase-9 and caspase-3 mediated apoptosis, and blocked the overexpression of extracellular signal-regulated kinase (ERK). We observed significantly greater intracellular accumulation of Cd and reduced expression of divalent metal transporter 1 (DMT1) protein in the heterozygous HTT striatal cells upon Cd exposure. Treatment with zinc, manganese, and iron as well as exogenous antioxidants significantly attenuated the Cd-induced cytotoxicity. Collectively, these results demonstrate that heterozygous HTT exhibits greater neurotoxic properties when coupled with Cd exposure to cause cell death via caspase mediated apoptosis, altered metal transport, and modulation of ERK and PKCδ dependent oxidative signaling mechanisms.
中文翻译:

杂合亨廷顿蛋白通过改变金属转运和蛋白激酶Cδ依赖性氧化应激和凋亡信号传导机制促进纹状体细胞中镉的神经毒性和神经变性
亨廷顿舞蹈病(HD)在功能上与环境因素相关,包括吸烟和金属水平的动态平衡。有趣的是,香烟中最丰富的重金属之一是镉(Cd),镉也堆积在纹状体中,并在暴露时引起神经毒性。因此,我们假设,负责患者大多数HD病例的杂合亨廷顿蛋白(HTT)与Cd接触会通过增加Cd在细胞内的蓄积和激活小鼠纹状体细胞中的氧化应激信号传导机制而引起神经毒性和神经退行性变。高清线模型。我们报告说,与野生型HTT细胞接触48小时后,杂合HTT纹状体细胞对镉诱导的细胞毒性的敏感性更高。
杂合的HTT和Cd诱导的细胞毒性导致NADPH氧化酶(NOX)介导的氧化应激被外源抗氧化剂和NOX抑制剂Apocynin减弱。杂合HTT结合Cd暴露导致蛋白激酶Cδ(PKCδ)和其他关键氧化应激蛋白水平的表达增加,增强了caspase-9和caspase-3介导的细胞凋亡的激活,并阻止了细胞外信号调节激酶的过表达( ERK)。我们观察到Cd暴露后,杂合HTT纹状体细胞中Cd的细胞内积累明显增加,二价金属转运蛋白1(DMT1)蛋白的表达降低。用锌,锰和铁以及外源性抗氧化剂处理可大大减轻Cd诱导的细胞毒性。总的来说,



























 京公网安备 11010802027423号
京公网安备 11010802027423号