当前位置:
X-MOL 学术
›
Mucosal Immunol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Entamoeba histolytica-induced IL-1β secretion is dependent on caspase-4 and gasdermin D.
Mucosal Immunology ( IF 8 ) Pub Date : 2018-Oct-25 , DOI: 10.1038/s41385-018-0101-9 Jeanie Quach 1 , France Moreau 1 , Christina Sandall 1 , Kris Chadee 1
Mucosal Immunology ( IF 8 ) Pub Date : 2018-Oct-25 , DOI: 10.1038/s41385-018-0101-9 Jeanie Quach 1 , France Moreau 1 , Christina Sandall 1 , Kris Chadee 1
Affiliation
|
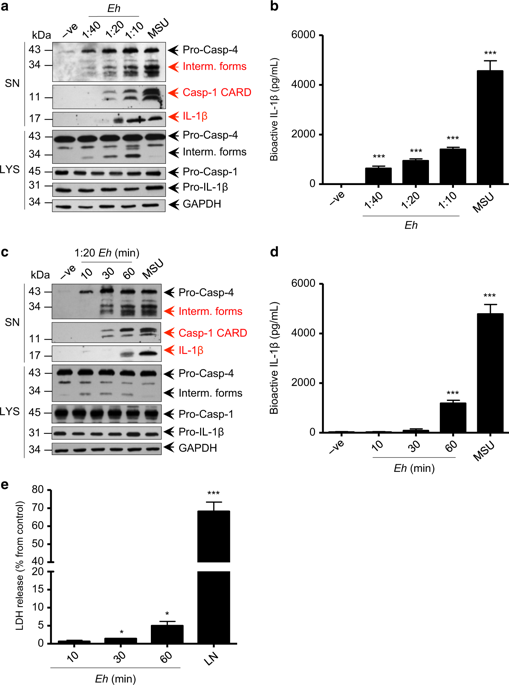
During invasion, Entamoeba histolytica (Eh) encounter macrophages and activate them to elicit tissue damaging pro-inflammatory responses. When Eh binds macrophages via the Gal-lectin, surface EhCP-A5 RGD sequence ligates α5β1 integrin to activate caspase-1 in a complex known as the NLRP3 inflammasome. In this study, we investigated Eh requirements underlying macrophage caspase-4 and -1 activation and the role caspase-4 and gasdermin D (GSDMD) play in augmenting pro-inflammatory cytokine responses. Caspase-4 activation was similar to caspase-1 requiring live Eh attachment via the Gal-lectin and EhCP-A5. However, unlike caspase-1, caspase-4 activation was independent of ASC and NLRP3. Using CRISPR/Cas9 gene editing of caspase-4 and -1 and GSDMD, we determined that caspase-1 and bioactive IL-1β release was highly dependent on caspase-4 activation and cleavage of GSDMD in response to Eh. Formaldehyde cross-linking to stabilize protein-protein interactions in transfected COS-7 cells stimulated with Eh revealed that caspase-4 specifically interacted with caspase-1 in a protein complex that enhanced the cleavage of caspase-1 CARD domains to augment IL-1β release. Activated caspase-4 and -1 cleaved GSDMD liberating the N-terminal p30 pore-forming fragment that caused the secretion of IL-1β. These findings reveal a novel role for caspase-4 as a sensor molecule to amplify pro-inflammatory responses when macrophage encounters Eh.
中文翻译:

溶组织内阿米巴诱导的 IL-1β 分泌依赖于 caspase-4 和 gasdermin D。
在入侵期间,溶组织内阿米巴 (Eh) 遇到巨噬细胞并激活它们以引发组织破坏性促炎反应。当 Eh 通过半乳糖凝集素结合巨噬细胞时,表面 EhCP-A5 RGD 序列连接 α 5 β 1整合素激活称为 NLRP3 炎性体的复合物中的 caspase-1。在这项研究中,我们调查了巨噬细胞 caspase-4 和 -1 激活的 Eh 需求以及 caspase-4 和 gasdermin D (GSDMD) 在增强促炎细胞因子反应中的作用。Caspase-4 激活类似于 caspase-1,需要通过半乳糖凝集素和 EhCP-A5 进行活 Eh 附着。然而,与 caspase-1 不同的是,caspase-4 的激活独立于 ASC 和 NLRP3。使用 caspase-4 和 -1 以及 GSDMD 的 CRISPR/Cas9 基因编辑,我们确定 caspase-1 和生物活性 IL-1β 释放高度依赖于 caspase-4 激活和 GSDMD 响应 Eh 的裂解。甲醛交联以稳定用 Eh 刺激的转染 COS-7 细胞中的蛋白质-蛋白质相互作用表明 caspase-4 与蛋白质复合物中的 caspase-1 特异性相互作用,增强 caspase-1 CARD 结构域的切割以增加 IL-1β 释放. 活化的 caspase-4 和 -1 裂解 GSDMD,释放导致 IL-1β 分泌的 N 末端 p30 成孔片段。这些发现揭示了 caspase-4 作为传感器分子的新作用,可在巨噬细胞遇到 Eh 时放大促炎反应。
更新日期:2019-01-26
中文翻译:

溶组织内阿米巴诱导的 IL-1β 分泌依赖于 caspase-4 和 gasdermin D。
在入侵期间,溶组织内阿米巴 (Eh) 遇到巨噬细胞并激活它们以引发组织破坏性促炎反应。当 Eh 通过半乳糖凝集素结合巨噬细胞时,表面 EhCP-A5 RGD 序列连接 α 5 β 1整合素激活称为 NLRP3 炎性体的复合物中的 caspase-1。在这项研究中,我们调查了巨噬细胞 caspase-4 和 -1 激活的 Eh 需求以及 caspase-4 和 gasdermin D (GSDMD) 在增强促炎细胞因子反应中的作用。Caspase-4 激活类似于 caspase-1,需要通过半乳糖凝集素和 EhCP-A5 进行活 Eh 附着。然而,与 caspase-1 不同的是,caspase-4 的激活独立于 ASC 和 NLRP3。使用 caspase-4 和 -1 以及 GSDMD 的 CRISPR/Cas9 基因编辑,我们确定 caspase-1 和生物活性 IL-1β 释放高度依赖于 caspase-4 激活和 GSDMD 响应 Eh 的裂解。甲醛交联以稳定用 Eh 刺激的转染 COS-7 细胞中的蛋白质-蛋白质相互作用表明 caspase-4 与蛋白质复合物中的 caspase-1 特异性相互作用,增强 caspase-1 CARD 结构域的切割以增加 IL-1β 释放. 活化的 caspase-4 和 -1 裂解 GSDMD,释放导致 IL-1β 分泌的 N 末端 p30 成孔片段。这些发现揭示了 caspase-4 作为传感器分子的新作用,可在巨噬细胞遇到 Eh 时放大促炎反应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号