当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kinetic Influence of Surface Charge Transfer Reactions Preceded by Non‐Electrochemical Processes on the Response in Cyclic Voltammetry
ChemElectroChem ( IF 4 ) Pub Date : 2018-11-29 , DOI: 10.1002/celc.201801275 Joaquin Gonzalez 1 , Francisco Martinez-Ortiz 1 , Encarnacion Torralba 2 , Angela Molina 1
ChemElectroChem ( IF 4 ) Pub Date : 2018-11-29 , DOI: 10.1002/celc.201801275 Joaquin Gonzalez 1 , Francisco Martinez-Ortiz 1 , Encarnacion Torralba 2 , Angela Molina 1
Affiliation
|
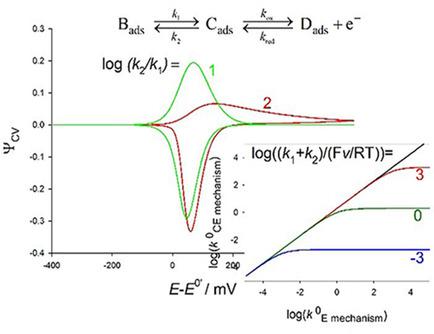
A general analytical explicit expression for the response of a surface charge transfer preceded by a non‐electrochemical process has been deduced. This solution is applicable to any electrochemical technique independent of the rates of both reactions. Interesting simplified cases are obtained for very common experimental situations in which the rates of electrochemical and non‐electrochemical steps are similar. These expressions have been applied to cyclic voltammetry in order to analyze the kinetic influences on the current‐potential response and to propose procedures for determining the rate and equilibrium constants of the mechanism. Moreover, it has been obtained an “apparent” rate constant of the overall process that reduces to a pure non‐electrochemical rate‐limiting process or to a pure electrochemical rate limiting one, under the right conditions. Experimental verification of the theoretical framework has been done with the analysis of the host‐guest complexation between a surface confined electroactive ferrocene moiety and a solution soluble cyclodextrin in a water‐ethanol media. From the dynamic analysis of the influence of the scan rate on the cyclic voltammetry curves, values have been obtained for the binding constant ( ) and rate constants for the inclusion complex formation and dissociation showing the great stability of the electro‐inactive ferrocene‐cyclodextrin complex.
) and rate constants for the inclusion complex formation and dissociation showing the great stability of the electro‐inactive ferrocene‐cyclodextrin complex.
中文翻译:

非电化学过程之前的表面电荷转移反应对循环伏安法响应的动力学影响
推导了在非电化学过程之前对表面电荷转移的响应的一般分析显式表达式。该解决方案适用于任何与两个反应速率无关的电化学技术。对于电化学和非电化学步骤的速率相似的非常常见的实验情况,可以得到有趣的简化案例。这些表达式已应用于循环伏安法,以分析动力学对电流电势响应的影响,并提出确定该机理的速率和平衡常数的程序。而且,已经获得了整个过程的“表观”速率常数,该常数降低为纯粹的非电化学速率限制过程或纯粹的电化学速率限制过程,在适当的条件下。通过分析表面受限的电活性二茂铁部分与水-乙醇介质中的可溶性环糊精之间的主客体络合,对理论框架进行了实验验证。通过动态分析扫描速率对循环伏安曲线的影响,获得了结合常数的值( )和包合物形成和解离的速率常数,表明电惰性二茂铁-环糊精复合物具有很高的稳定性。
)和包合物形成和解离的速率常数,表明电惰性二茂铁-环糊精复合物具有很高的稳定性。
更新日期:2018-11-29
 ) and rate constants for the inclusion complex formation and dissociation showing the great stability of the electro‐inactive ferrocene‐cyclodextrin complex.
) and rate constants for the inclusion complex formation and dissociation showing the great stability of the electro‐inactive ferrocene‐cyclodextrin complex.
中文翻译:

非电化学过程之前的表面电荷转移反应对循环伏安法响应的动力学影响
推导了在非电化学过程之前对表面电荷转移的响应的一般分析显式表达式。该解决方案适用于任何与两个反应速率无关的电化学技术。对于电化学和非电化学步骤的速率相似的非常常见的实验情况,可以得到有趣的简化案例。这些表达式已应用于循环伏安法,以分析动力学对电流电势响应的影响,并提出确定该机理的速率和平衡常数的程序。而且,已经获得了整个过程的“表观”速率常数,该常数降低为纯粹的非电化学速率限制过程或纯粹的电化学速率限制过程,在适当的条件下。通过分析表面受限的电活性二茂铁部分与水-乙醇介质中的可溶性环糊精之间的主客体络合,对理论框架进行了实验验证。通过动态分析扫描速率对循环伏安曲线的影响,获得了结合常数的值(
 )和包合物形成和解离的速率常数,表明电惰性二茂铁-环糊精复合物具有很高的稳定性。
)和包合物形成和解离的速率常数,表明电惰性二茂铁-环糊精复合物具有很高的稳定性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号