Bone Research ( IF 12.7 ) Pub Date : 2018-10-17 , DOI: 10.1038/s41413-018-0031-x Yuxing Guo , Yuan Yuan , Ling Wu , Thach-Vu Ho , Junjun Jing , Hideki Sugii , Jingyuan Li , Xia Han , Jifan Feng , Chuanbin Guo , Yang Chai
|
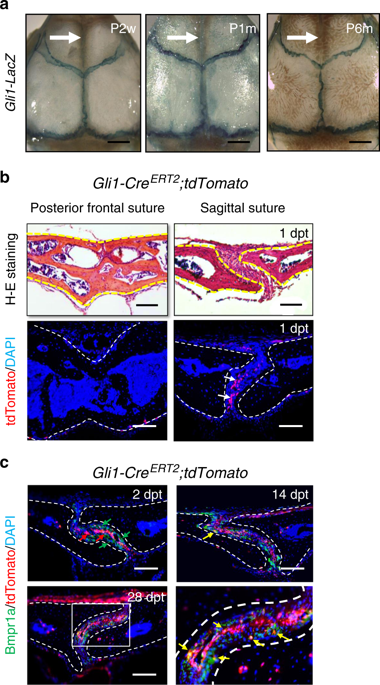
Calvarial bones are connected by fibrous sutures. These sutures provide a niche environment that includes mesenchymal stem cells (MSCs), osteoblasts, and osteoclasts, which help maintain calvarial bone homeostasis and repair. Abnormal function of osteogenic cells or diminished MSCs within the cranial suture can lead to skull defects, such as craniosynostosis. Despite the important function of each of these cell types within the cranial suture, we have limited knowledge about the role that crosstalk between them may play in regulating calvarial bone homeostasis and injury repair. Here we show that suture MSCs give rise to osteoprogenitors that show active bone morphogenetic protein (BMP) signalling and depend on BMP-mediated Indian hedgehog (IHH) signalling to balance osteogenesis and osteoclastogenesis activity. IHH signalling and receptor activator of nuclear factor kappa-Β ligand (RANKL) may function synergistically to promote the differentiation and resorption activity of osteoclasts. Loss of Bmpr1a in MSCs leads to downregulation of hedgehog (Hh) signalling and diminished cranial sutures. Significantly, activation of Hh signalling partially restores suture morphology in Bmpr1a mutant mice, suggesting the functional importance of BMP-mediated Hh signalling in regulating suture tissue homeostasis. Furthermore, there is an increased number of CD200+ cells in Bmpr1a mutant mice, which may also contribute to the inhibited osteoclast activity in the sutures of mutant mice. Finally, suture MSCs require BMP-mediated Hh signalling during the repair of calvarial bone defects after injury. Collectively, our studies reveal the molecular and cellular mechanisms governing cell–cell interactions within the cranial suture that regulate calvarial bone homeostasis and repair.
中文翻译:

BMP-IHH介导的间充质干细胞与破骨细胞之间的相互作用支持颅骨稳态和修复
颅骨通过纤维缝合线相连。这些缝合线提供了一个利基环境,其中包括间充质干细胞(MSC),成骨细胞和破骨细胞,这有助于维持颅骨稳态和修复。颅骨缝线内成骨细胞功能异常或MSCs减少可导致颅骨缺损,例如颅突。尽管颅内缝线中每种细胞类型都起着重要的作用,但我们对它们之间的串扰在调节颅骨稳态和损伤修复中所起的作用的知识知之甚少。在这里,我们显示缝合线MSC产生骨祖细胞,该骨祖细胞显示出活性的骨形态发生蛋白(BMP)信号,并依赖BMP介导的印度刺猬(IHH)信号来平衡成骨和破骨细胞活性。IHH信号和核因子κ-β配体(RANKL)的受体激活剂可能具有协同作用,以促进破骨细胞的分化和吸收活性。的损失MSC中的Bmpr1a导致刺猬(Hh)信号的下调和颅骨缝线的减少。重要的是,Hh信号的激活部分恢复了Bmpr1a突变小鼠的缝线形态,表明BMP介导的Hh信号在调节缝线组织动态平衡中的功能重要性。此外,Bmpr1a突变小鼠中CD200 +细胞的数量增加,这也可能有助于突变小鼠缝线中破骨细胞活性的抑制。最后,缝线间充质干细胞在损伤后颅骨缺损修复过程中需要BMP介导的Hh信号传导。总的来说,我们的研究揭示了控制颅骨缝线内细胞间相互作用的分子和细胞机制,这些机制调节颅骨的稳态和修复。


























 京公网安备 11010802027423号
京公网安备 11010802027423号