npj Regenerative Medicine ( IF 7.2 ) Pub Date : 2018-10-11 , DOI: 10.1038/s41536-018-0055-2 Takaharu Negoro , Yuri Takagaki , Hanayuki Okura , Akifumi Matsuyama
|
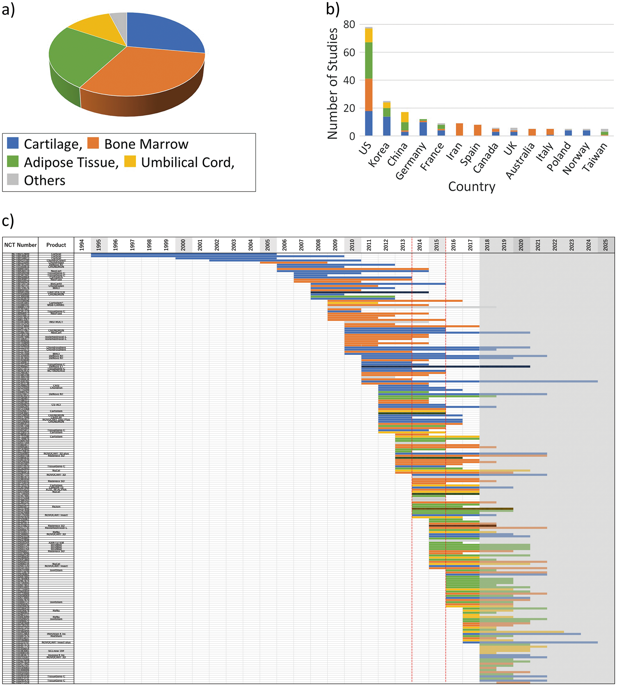
Focal and degenerative lesions of articular cartilage greatly reduce the patient’s quality of life. Various therapies including surgical treatment have been developed, but a definitive therapy is not yet known. Several cell therapy products have already been developed and are available in the market. In this study, we examined the clinical research trends related to cell therapy products in the cartilage repair field based on data obtained from the ClinicalTrial.gov website. Although this website does not provide comprehensive results of clinical trials, it offers information on prospective clinical trials, including work in progress, and thus allows for chronological analysis of the data. We selected 203 studies related to the field of cartilage regeneration from ClinicalTrial.gov. The results showed a shift in the clinical translational trend in utilized cells from cartilage- and bone marrow- to adipose tissue-based cells. Whereas the studies that used cartilage as the cell source included many phase III trials, fewer studies using bone marrow and adipose tissue cells progressed to phase III, suggesting that most clinical developments using the latter sources have not been successful so far. One product covered the entire period from the start of phase I to the completion of phase III, with a time to completion of more than 100 months. Translational trends in autologous chondrocyte implantation were also discussed. The use of ClinicalTrials.gov as the sole data source can yield a perspective view of the global clinical translational trends, which has been difficult to observe up to this point.
中文翻译:

细胞疗法修复关节软骨的临床试验趋势
关节软骨的局灶性和退行性病变大大降低了患者的生活质量。已经开发出包括外科手术治疗在内的各种疗法,但是确切的疗法尚不为人所知。已经开发了几种细胞疗法产品,并且可以在市场上买到。在这项研究中,我们根据从ClinicalTrial.gov网站获得的数据,研究了软骨修复领域中与细胞治疗产品相关的临床研究趋势。尽管此网站未提供临床试验的全面结果,但它提供了有关前瞻性临床试验的信息,包括进行中的工作,因此可以按时间顺序对数据进行分析。我们从ClinicalTrial.gov中选择了203个与软骨再生领域相关的研究。结果表明,所用细胞的临床翻译趋势已从软骨和骨髓转变为脂肪组织为基础的细胞。尽管使用软骨作为细胞来源的研究包括许多III期试验,但使用骨髓和脂肪组织细胞进行到III期的研究较少,这表明迄今为止使用后者来源进行的大多数临床开发均未成功。一种产品涵盖了从第一阶段开始到第三阶段结束的整个时期,完成时间超过100个月。还讨论了自体软骨细胞植入的翻译趋势。使用ClinicalTrials.gov作为唯一的数据源可以提供全球临床翻译趋势的透视图,到目前为止,这一点很难观察到。


























 京公网安备 11010802027423号
京公网安备 11010802027423号