当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Nitrosopyridine Probe To Detect Polyketide Natural Products with Conjugated Alkenes: Discovery of Novodaryamide and Nocarditriene
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-10-01 00:00:00 , DOI: 10.1021/acschembio.8b00598 Gabriel Castro-Falcón 1 , Natalie Millán-Aguiñaga 1 , Catherine Roullier 2 , Paul R. Jensen 1 , Chambers C. Hughes 1
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-10-01 00:00:00 , DOI: 10.1021/acschembio.8b00598 Gabriel Castro-Falcón 1 , Natalie Millán-Aguiñaga 1 , Catherine Roullier 2 , Paul R. Jensen 1 , Chambers C. Hughes 1
Affiliation
|
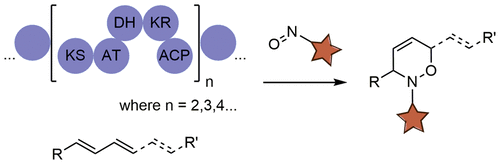
An optimized nitroso-based probe that facilitates the discovery of conjugated alkene-containing natural products in unprocessed extracts was developed. It chemoselectively reacts with conjugated olefins via a nitroso-Diels–Alder cyclization to yield derivatives with a distinct chromophore and an isotopically unique bromine atom that can be rapidly identified using liquid chromatography/mass spectrometry and a bioinformatics tool called MeHaloCoA (Marine Halogenated Compound Analysis). The probe is ideally employed when genome-mining techniques identify strains containing polyketide gene clusters with two or more repeating KS-AT-DH-KR-ACP domain sequences, which are required for the biosynthesis of conjugated alkenes. Comparing the reactivity and spectral properties of five brominated arylnitroso reagents with model compounds spiramycin, bufalin, rapamycin, and rifampicin led to the identification of 5-bromo-2-nitrosopyridine as the most suitable probe structure. The utility of the dienophile probe was then demonstrated in bacterial extracts. Tylactone, novodaryamide and daryamide A, piperazimycin A, and the saccharamonopyrones A and B were cleanly labeled in extracts from their respective bacterial producers, in high regioselectivity but with varying degrees of diastereoselectivity. Further application of the method led to the discovery of a new natural product called nocarditriene, containing an unprecedented epoxy-2,3,4,5-tetrahydropyridine structure, from marine-derived Nocardiopsis strain CNY-503.
中文翻译:

亚硝基吡啶探针可检测带有共轭烯烃的聚酮天然产物:新戊酰胺和诺卡三烯的发现
开发了一种优化的基于亚硝基的探针,该探针有助于在未加工的提取物中发现含共轭烯烃的天然产物。它通过亚硝基-Diels-Alder环化与共轭烯烃发生化学选择性反应,生成具有独特发色团和同位素独特溴原子的衍生物,可以使用液相色谱/质谱和称为MeHaloCoA(海洋卤代化合物分析)的生物信息学工具快速鉴定出衍生物。 。当基因组挖掘技术确定含有带有两个或多个重复KS-AT-DH-KR-ACP结构域序列的聚酮化合物基因簇的菌株时,该探针是理想的探针,这是共轭烯烃生物合成所必需的。比较五种溴代芳基亚硝基试剂与模型化合物spiramycin,bufalin,雷帕霉素和利福平导致鉴定出5-溴-2-亚硝基吡啶为最合适的探针结构。然后在细菌提取物中证明了亲双烯体探针的效用。在其各自细菌生产者的提取物中,以较高的区域选择性但非对映选择性不同的方式,干净地标记了内酯,novodaryamide和daryamide A,哌嗪霉素A和saccharamonopyrones A和B。该方法的进一步应用导致发现了一种新的天然产物,称为nocarditriene,其含有来自海洋的,前所未有的环氧2,3,4,5-四氢吡啶结构 novodaryamide和daryamide A,piperazimycin A和saccharamonopyrones A和B在其各自细菌生产者的提取物中进行了清晰的标记,具有较高的区域选择性,但具有不同程度的非对映选择性。该方法的进一步应用导致发现了一种新的天然产物,称为nocarditriene,其含有来自海洋的,前所未有的环氧2,3,4,5-四氢吡啶结构 novodaryamide和daryamide A,piperazimycin A和saccharamonopyrones A和B在其各自细菌生产者的提取物中进行了清晰的标记,具有较高的区域选择性,但具有不同程度的非对映选择性。该方法的进一步应用导致发现了一种新的天然产物,称为nocarditriene,其含有来自海洋的,前所未有的环氧2,3,4,5-四氢吡啶结构诺卡氏菌菌株CNY-503。
更新日期:2018-10-01
中文翻译:

亚硝基吡啶探针可检测带有共轭烯烃的聚酮天然产物:新戊酰胺和诺卡三烯的发现
开发了一种优化的基于亚硝基的探针,该探针有助于在未加工的提取物中发现含共轭烯烃的天然产物。它通过亚硝基-Diels-Alder环化与共轭烯烃发生化学选择性反应,生成具有独特发色团和同位素独特溴原子的衍生物,可以使用液相色谱/质谱和称为MeHaloCoA(海洋卤代化合物分析)的生物信息学工具快速鉴定出衍生物。 。当基因组挖掘技术确定含有带有两个或多个重复KS-AT-DH-KR-ACP结构域序列的聚酮化合物基因簇的菌株时,该探针是理想的探针,这是共轭烯烃生物合成所必需的。比较五种溴代芳基亚硝基试剂与模型化合物spiramycin,bufalin,雷帕霉素和利福平导致鉴定出5-溴-2-亚硝基吡啶为最合适的探针结构。然后在细菌提取物中证明了亲双烯体探针的效用。在其各自细菌生产者的提取物中,以较高的区域选择性但非对映选择性不同的方式,干净地标记了内酯,novodaryamide和daryamide A,哌嗪霉素A和saccharamonopyrones A和B。该方法的进一步应用导致发现了一种新的天然产物,称为nocarditriene,其含有来自海洋的,前所未有的环氧2,3,4,5-四氢吡啶结构 novodaryamide和daryamide A,piperazimycin A和saccharamonopyrones A和B在其各自细菌生产者的提取物中进行了清晰的标记,具有较高的区域选择性,但具有不同程度的非对映选择性。该方法的进一步应用导致发现了一种新的天然产物,称为nocarditriene,其含有来自海洋的,前所未有的环氧2,3,4,5-四氢吡啶结构 novodaryamide和daryamide A,piperazimycin A和saccharamonopyrones A和B在其各自细菌生产者的提取物中进行了清晰的标记,具有较高的区域选择性,但具有不同程度的非对映选择性。该方法的进一步应用导致发现了一种新的天然产物,称为nocarditriene,其含有来自海洋的,前所未有的环氧2,3,4,5-四氢吡啶结构诺卡氏菌菌株CNY-503。


























 京公网安备 11010802027423号
京公网安备 11010802027423号