Developmental Cell ( IF 11.8 ) Pub Date : 2018-09-27 , DOI: 10.1016/j.devcel.2018.09.002 Mahmoud Abdul Karim , Erin Kate McNally , Dieter Ronny Samyn , Sevan Mattie , Christopher Leonard Brett
|
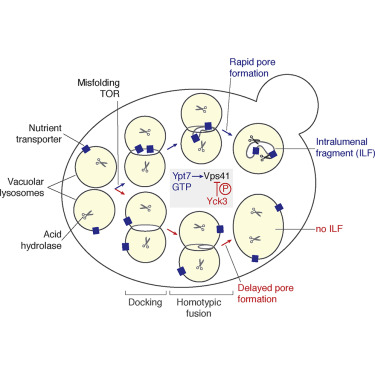
Upon vacuolar lysosome (or vacuole) fusion in S. cerevisiae, a portion of membrane is internalized and catabolized. Formation of this intralumenal fragment (ILF) is important for organelle protein and lipid homeostasis and remodeling. But how ILF formation is optimized for membrane turnover is not understood. Here, we show that fewer ILFs form when the interaction between the Rab-GTPase Ypt7 and its effector Vps41 (a subunit of the tethering complex HOPS) is interrupted by a point mutation (Ypt7-D44N). Subsequent phosphorylation of Vps41 by the casein kinase Yck3 prevents stabilization of trans-SNARE complexes needed for lipid bilayer pore formation. Impairing ILF formation prevents clearance of misfolded proteins from vacuole membranes and promotes organelle permeability and cell death. We propose that HOPS coordinates Rab, kinase, and SNARE cycles to modulate ILF size during vacuole fusion, regulating lipid and protein turnover important for quality control and membrane integrity.
中文翻译:

Rab-效应子-激酶相互作用调节液泡融合过程中内部片段的形成。
在酿酒酵母中液泡溶酶体(或液泡)融合后,一部分膜被内化和分解代谢。该管腔内片段(ILF)的形成对于细胞器蛋白质和脂质稳态和重塑非常重要。但是,如何针对膜更新优化ILF的形成尚不清楚。在这里,我们显示当Rab-GTPase Ypt7及其效应子Vps41(系留复合物HOPS的一个亚基)之间的相互作用被点突变(Ypt7-D44N)中断时,形成的ILF较少。酪蛋白激酶Yck3随后对Vps41的磷酸化阻止了脂质双层孔形成所需的反式-SNARE复合物的稳定。ILF形成受损会阻止错误折叠的蛋白质从液泡膜中清除,并促进细胞器通透性和细胞死亡。我们建议HOPS协调Rab,激酶和SNARE周期,以在液泡融合过程中调节ILF的大小,



























 京公网安备 11010802027423号
京公网安备 11010802027423号