当前位置:
X-MOL 学术
›
Environ. Sci. Technol. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Calcium Silicate Crystal Structure Impacts Reactivity with CO2 and Precipitate Chemistry
Environmental Science & Technology Letters ( IF 10.9 ) Pub Date : 2018-08-29 , DOI: 10.1021/acs.estlett.8b00386 Dan A. Plattenberger 1 , Florence T. Ling 2 , Zhiyuan Tao 1 , Catherine A. Peters 2 , Andres F. Clarens 1
Environmental Science & Technology Letters ( IF 10.9 ) Pub Date : 2018-08-29 , DOI: 10.1021/acs.estlett.8b00386 Dan A. Plattenberger 1 , Florence T. Ling 2 , Zhiyuan Tao 1 , Catherine A. Peters 2 , Andres F. Clarens 1
Affiliation
|
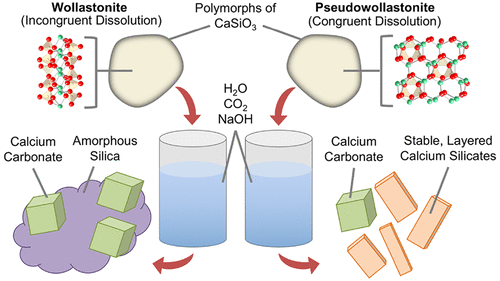
The reaction of CO2(aq) with calcium silicates creates precipitates that can impact fluid flow in subsurface applications such as geologic CO2 storage and geothermal energy. These reactions nominally produce calcium carbonate (CaCO3) and amorphous silica (SiOx). Here we report evidence that the crystal structure of the parent silicate determines the way in which it reacts with CO2 and the resulting structures of the reaction products. Batch experiments were performed using two polymorphs of a model calcium silicate (CaSiO3), wollastonite (chain-structured) and pseudowollastonite (ring-structured), at elevated temperatures (150 °C) and partial pressures of CO2 (0–11 MPa). Reaction of CO2(aq) with wollastonite produced CaCO3 and SiOx, whereas reaction of CO2(aq) with pseudowollastonite produced platelike crystalline calcium silicate phases, along with CaCO3 and SiOx. A reaction mechanism that explains the observations in relation to dissolution of the parent silicate, the pH of the solution, and the presence of nucleation sites is proposed. The mechanism is supported with inductively coupled plasma optical emission spectrometry measurements and scanning electron microscopy/transmission electron microscopy–selected-area electron diffraction characterization of solid products. These findings are important for a number of reasons, among them, the fact that the crystalline silicate precipitates are more stable than CaCO3 under low-pH conditions, which could be valuable for creating permanent seals in subsurface applications.
中文翻译:

硅酸钙晶体结构影响与CO 2和沉淀化学反应
CO 2(aq)与硅酸钙的反应产生沉淀,这些沉淀会影响地下应用(例如地质CO 2储存和地热能)中的流体流动。这些反应通常产生碳酸钙(CaCO 3)和无定形二氧化硅(SiO x)。在这里我们报告的证据表明,母体硅酸盐的晶体结构决定了它与CO 2反应的方式以及反应产物的结构。使用模型硅酸钙(CaSiO 3),硅灰石(链结构)和假硅灰石(环结构)的两种多晶型物,在升高的温度(150°C)和CO 2分压下进行了批量实验。(0-11 MPa)。CO 2(aq)与硅灰石的反应生成CaCO 3和SiO x,而CO 2(aq)与假硅灰石的反应生成板状结晶硅酸钙相以及CaCO 3和SiO x。提出了一种反应机理,该机理解释了与母体硅酸盐的溶解,溶液的pH和成核位点有关的观察结果。该机制由电感耦合等离子体发射光谱测量和固体产品的扫描电子显微镜/透射电子显微镜选择区域电子衍射表征支持。这些发现很重要,其原因有很多,其中包括在低pH条件下结晶硅酸盐沉淀比CaCO 3稳定的事实,这对于在地下应用中形成永久密封非常有价值。
更新日期:2018-08-30
中文翻译:

硅酸钙晶体结构影响与CO 2和沉淀化学反应
CO 2(aq)与硅酸钙的反应产生沉淀,这些沉淀会影响地下应用(例如地质CO 2储存和地热能)中的流体流动。这些反应通常产生碳酸钙(CaCO 3)和无定形二氧化硅(SiO x)。在这里我们报告的证据表明,母体硅酸盐的晶体结构决定了它与CO 2反应的方式以及反应产物的结构。使用模型硅酸钙(CaSiO 3),硅灰石(链结构)和假硅灰石(环结构)的两种多晶型物,在升高的温度(150°C)和CO 2分压下进行了批量实验。(0-11 MPa)。CO 2(aq)与硅灰石的反应生成CaCO 3和SiO x,而CO 2(aq)与假硅灰石的反应生成板状结晶硅酸钙相以及CaCO 3和SiO x。提出了一种反应机理,该机理解释了与母体硅酸盐的溶解,溶液的pH和成核位点有关的观察结果。该机制由电感耦合等离子体发射光谱测量和固体产品的扫描电子显微镜/透射电子显微镜选择区域电子衍射表征支持。这些发现很重要,其原因有很多,其中包括在低pH条件下结晶硅酸盐沉淀比CaCO 3稳定的事实,这对于在地下应用中形成永久密封非常有价值。


























 京公网安备 11010802027423号
京公网安备 11010802027423号