当前位置:
X-MOL 学术
›
Integr. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Single-molecule tracking in live Yersinia enterocolitica reveals distinct cytosolic complexes of injectisome subunits
Integrative Biology ( IF 2.5 ) Pub Date : 2018-08-13 , DOI: 10.1039/c8ib00075a Julian Michael Rocha 1, 2, 3 , Charles Joseph Richardson 1, 2, 3 , Mingxing Zhang 1, 2, 3 , Caroline Maureen Darch 1, 2, 3 , Eugene Cai 1, 2, 3 , Andreas Diepold 4, 5, 6, 7 , Andreas Gahlmann 1, 2, 2, 3, 8
Integrative Biology ( IF 2.5 ) Pub Date : 2018-08-13 , DOI: 10.1039/c8ib00075a Julian Michael Rocha 1, 2, 3 , Charles Joseph Richardson 1, 2, 3 , Mingxing Zhang 1, 2, 3 , Caroline Maureen Darch 1, 2, 3 , Eugene Cai 1, 2, 3 , Andreas Diepold 4, 5, 6, 7 , Andreas Gahlmann 1, 2, 2, 3, 8
Affiliation
|
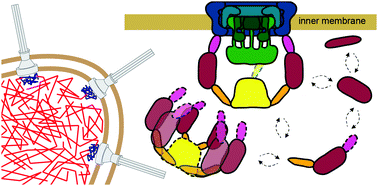
In bacterial type 3 secretion, substrate proteins are actively transported from the bacterial cytoplasm into the host cell cytoplasm by a large membrane-embedded machinery called the injectisome. Injectisomes transport secretion substrates in response to specific environmental signals, but the molecular details by which the cytosolic secretion substrates are selected and transported through the type 3 secretion pathway remain unclear. Secretion activity and substrate selectivity are thought to be controlled by a sorting platform consisting of the proteins SctK, SctQ, SctL, and SctN, which together localize to the cytoplasmic side of membrane-embedded injectisomes. However, recent work revealed that sorting platform proteins additionally exhibit substantial cytosolic populations and that SctQ reversibly binds to and dissociates from the cytoplasmic side of membrane-embedded injectisomes. Based on these observations, we hypothesized that dynamic molecular turnover at the injectisome and cytosolic assembly among sorting platform proteins is a critical regulatory component of type 3 secretion. To determine whether sorting platform complexes exist in the cytosol, we measured the diffusive properties of the two central sorting platform proteins, SctQ and SctL, using live cell high-throughput 3D single-molecule tracking microscopy. Single-molecule trajectories, measured in wild-type and mutant Yersinia enterocolitica cells, reveal that both SctQ and SctL exist in several distinct diffusive states in the cytosol, indicating that these proteins form stable homo- and hetero-oligomeric complexes in their native environment. Our findings provide the first diffusive state-resolved insights into the dynamic regulatory network that interfaces stationary membrane-embedded injectisomes with the soluble cytosolic components of the type 3 secretion system.
中文翻译:

活小肠结肠炎耶尔森氏菌中的单分子跟踪揭示了注射体亚基的独特胞质复合物
在细菌的3型分泌中,底物蛋白通过称为注射小体的大型膜嵌入机制从细菌细胞质主动转运到宿主细胞的细胞质中。注射异构体响应特定的环境信号转运分泌底物,但尚不清楚选择胞质分泌底物并通过3型分泌途径转运的分子细节。分泌活性和底物选择性被认为是由蛋白质SctK,SctQ,SctL和SctN组成的分选平台控制的,它们共同位于膜嵌入注射异构体的细胞质侧。然而,最近的工作表明,分选平台蛋白还表现出大量的细胞溶质群,并且SctQ可逆地结合到膜包埋的注射异构体的细胞质侧并从其解离。基于这些观察,我们假设分选平台蛋白之间的注射体和胞质组装处的动态分子更新是3型分泌的关键调控成分。为了确定分选平台复合物是否存在于细胞质中,我们使用活细胞高通量3D单分子跟踪显微镜测量了两个中央分选平台蛋白SctQ和SctL的扩散特性。在野生型和突变型中测得的单分子轨迹 我们假设分选平台蛋白之间的注射体和胞质组装处的动态分子更新是3型分泌的关键调控成分。为了确定分选平台复合物是否存在于细胞质中,我们使用活细胞高通量3D单分子跟踪显微镜测量了两个中央分选平台蛋白SctQ和SctL的扩散特性。在野生型和突变型中测得的单分子轨迹 我们假设分选平台蛋白之间的注射体和胞质组装处的动态分子更新是3型分泌的关键调控成分。为了确定分选平台复合物是否存在于细胞质中,我们使用活细胞高通量3D单分子跟踪显微镜测量了两个中央分选平台蛋白SctQ和SctL的扩散特性。在野生型和突变型中测得的单分子轨迹小肠结肠炎耶尔森氏菌细胞显示,SctQ和SctL都以几种不同的扩散状态存在于细胞质中,表明这些蛋白质在其天然环境中形成稳定的同型和异型寡聚复合物。我们的发现为动态调节网络提供了第一个扩散状态解决的见解,该动态调节网络将固定膜嵌入的注射异构体与3型分泌系统的可溶性胞质组分相连接。
更新日期:2018-08-13
中文翻译:

活小肠结肠炎耶尔森氏菌中的单分子跟踪揭示了注射体亚基的独特胞质复合物
在细菌的3型分泌中,底物蛋白通过称为注射小体的大型膜嵌入机制从细菌细胞质主动转运到宿主细胞的细胞质中。注射异构体响应特定的环境信号转运分泌底物,但尚不清楚选择胞质分泌底物并通过3型分泌途径转运的分子细节。分泌活性和底物选择性被认为是由蛋白质SctK,SctQ,SctL和SctN组成的分选平台控制的,它们共同位于膜嵌入注射异构体的细胞质侧。然而,最近的工作表明,分选平台蛋白还表现出大量的细胞溶质群,并且SctQ可逆地结合到膜包埋的注射异构体的细胞质侧并从其解离。基于这些观察,我们假设分选平台蛋白之间的注射体和胞质组装处的动态分子更新是3型分泌的关键调控成分。为了确定分选平台复合物是否存在于细胞质中,我们使用活细胞高通量3D单分子跟踪显微镜测量了两个中央分选平台蛋白SctQ和SctL的扩散特性。在野生型和突变型中测得的单分子轨迹 我们假设分选平台蛋白之间的注射体和胞质组装处的动态分子更新是3型分泌的关键调控成分。为了确定分选平台复合物是否存在于细胞质中,我们使用活细胞高通量3D单分子跟踪显微镜测量了两个中央分选平台蛋白SctQ和SctL的扩散特性。在野生型和突变型中测得的单分子轨迹 我们假设分选平台蛋白之间的注射体和胞质组装处的动态分子更新是3型分泌的关键调控成分。为了确定分选平台复合物是否存在于细胞质中,我们使用活细胞高通量3D单分子跟踪显微镜测量了两个中央分选平台蛋白SctQ和SctL的扩散特性。在野生型和突变型中测得的单分子轨迹小肠结肠炎耶尔森氏菌细胞显示,SctQ和SctL都以几种不同的扩散状态存在于细胞质中,表明这些蛋白质在其天然环境中形成稳定的同型和异型寡聚复合物。我们的发现为动态调节网络提供了第一个扩散状态解决的见解,该动态调节网络将固定膜嵌入的注射异构体与3型分泌系统的可溶性胞质组分相连接。


























 京公网安备 11010802027423号
京公网安备 11010802027423号