当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Correlation between Cellular Uptake and Cytotoxicity of Fragmented α-Synuclein Amyloid Fibrils Suggests Intracellular Basis for Toxicity.
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2020-01-08 , DOI: 10.1021/acschemneuro.9b00562 Xiaolu Zhang 1 , Emelie Wesén 1 , Ranjeet Kumar 1 , David Bernson 1 , Audrey Gallud 1 , Alexandra Paul 1 , Pernilla Wittung-Stafshede 1 , Elin K Esbjörner 1
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2020-01-08 , DOI: 10.1021/acschemneuro.9b00562 Xiaolu Zhang 1 , Emelie Wesén 1 , Ranjeet Kumar 1 , David Bernson 1 , Audrey Gallud 1 , Alexandra Paul 1 , Pernilla Wittung-Stafshede 1 , Elin K Esbjörner 1
Affiliation
|
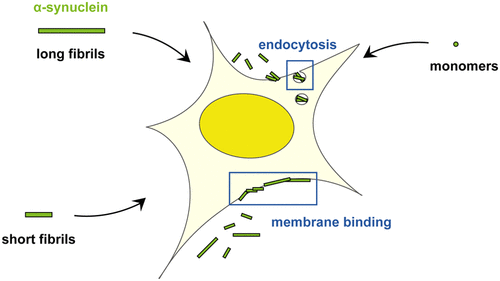
Aggregation and intracellular deposition of the protein α-synuclein is an underlying characteristic of Parkinson's disease. α-Synuclein assemblies also undergo cell-cell spreading, facilitating propagation of their cellular pathology. Understanding how cellular interactions and uptake of extracellular α-synuclein assemblies depend on their physical attributes is therefore important. We prepared fragmented fluorescently labeled α-synuclein amyloid fibrils of different average lengths (∼80 nm to >1 μm) and compared their interactions with SH-SY5Y cells. We report that fibrils of all lengths, but not monomers, bind avidly to the cell surface. Their uptake is inversely dependent on their average size, occurs via a heparan sulfate dependent endocytic route, and appears to have a size cutoff of ∼400 nm. The uptake of α-synuclein fibrils, but not monomers, correlates with their cytotoxicity as measured by reduction in metabolic activity, strongly suggesting an intracellular basis for α-synuclein fibril toxicity, likely involving endolysosomes.
中文翻译:

片段化的α-突触核蛋白淀粉样蛋白纤维的细胞摄取与细胞毒性之间的相关性表明细胞内毒性基础。
α-突触核蛋白的聚集和细胞内沉积是帕金森氏病的潜在特征。α-突触核蛋白组件也经历细胞间扩散,从而促进其细胞病理学的传播。因此,了解细胞相互作用和细胞外α-突触核蛋白装配的摄取如何取决于其物理属性非常重要。我们制备了不同平均长度(〜80 nm至> 1μm)的片段化的荧光标记的α-突触核蛋白淀粉样原纤维,并比较了它们与SH-SY5Y细胞的相互作用。我们报道,所有长度的原纤维,而不是单体,都狂热地结合到细胞表面。它们的摄取与它们的平均大小成反比,通过硫酸乙酰肝素依赖性的内吞途径发生,并且似乎具有约400nm的大小截止。α-突触核蛋白原纤维的摄取
更新日期:2020-01-09
中文翻译:

片段化的α-突触核蛋白淀粉样蛋白纤维的细胞摄取与细胞毒性之间的相关性表明细胞内毒性基础。
α-突触核蛋白的聚集和细胞内沉积是帕金森氏病的潜在特征。α-突触核蛋白组件也经历细胞间扩散,从而促进其细胞病理学的传播。因此,了解细胞相互作用和细胞外α-突触核蛋白装配的摄取如何取决于其物理属性非常重要。我们制备了不同平均长度(〜80 nm至> 1μm)的片段化的荧光标记的α-突触核蛋白淀粉样原纤维,并比较了它们与SH-SY5Y细胞的相互作用。我们报道,所有长度的原纤维,而不是单体,都狂热地结合到细胞表面。它们的摄取与它们的平均大小成反比,通过硫酸乙酰肝素依赖性的内吞途径发生,并且似乎具有约400nm的大小截止。α-突触核蛋白原纤维的摄取


























 京公网安备 11010802027423号
京公网安备 11010802027423号