Laboratory Investigation ( IF 5 ) Pub Date : 2020-01-02 , DOI: 10.1038/s41374-019-0365-z Shan-Zhong Yang 1 , Fei Xu 1, 2 , Kaiyu Yuan 1 , Yong Sun 1 , Tong Zhou 3 , Xinyang Zhao 4 , Jay M McDonald 1, 5 , Yabing Chen 1, 5
|
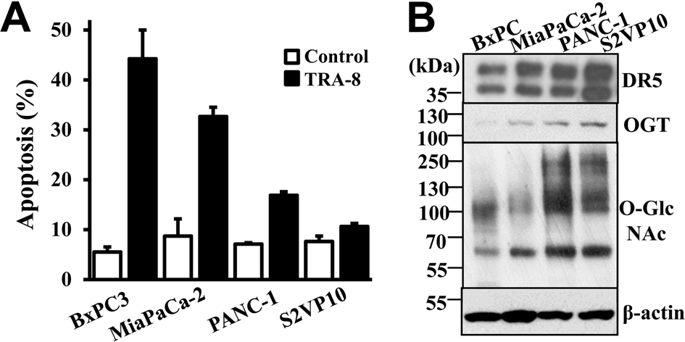
TRAIL-activating therapy is promising in treating various cancers, including pancreatic cancer, a highly malignant neoplasm with poor prognosis. However, many pancreatic cancer cells are resistant to TRAIL-induced apoptosis despite their expression of intact death receptors (DRs). Protein O-GlcNAcylation is a versatile posttranslational modification that regulates various biological processes. Elevated protein O-GlcNAcylation has been recently linked to cancer cell growth and survival. In this study, we evaluated the role of protein O-GlcNAcylation in pancreatic cancer TRAIL resistance, and identified higher levels of O-GlcNAcylation in TRAIL-resistant pancreatic cancer cells. With gain- and loss-of-function of the O-GlcNAc-adding enzyme, O-GlcNActransferase (OGT), we determined that increasing O-GlcNAcylation rendered TRAIL-sensitive cells more resistant to TRA-8-induced apoptosis, while inhibiting O-GlcNAcylation promoted TRA-8-induced apoptosis in TRAIL-resistance cells. Furthermore, we demonstrated that OGT knockdown sensitized TRAIL-resistant cells to TRA-8 therapy in a mouse model in vivo. Mechanistic studies revealed direct O-GlcNAc modifications of DR5, which regulated TRA-8-induced DR5 oligomerization. We further defined that DR5 O-GlcNAcylation was independent of FADD, the adapter protein for the downstream death-inducing signaling. These studies have demonstrated an important role of protein O-GlcNAcylation in regulating TRAIL resistance of pancreatic cancer cells; and uncovered the contribution of O-GlcNAcylation to DR5 oligomerization and thus mediating DR-inducing signaling.
中文翻译:

通过蛋白质 O-GlcNAc 酰化调节胰腺癌 TRAIL 耐药性
TRAIL 激活疗法在治疗各种癌症方面具有广阔的前景,包括胰腺癌,一种预后不良的高度恶性肿瘤。然而,许多胰腺癌细胞尽管表达完整的死亡受体(DR),但仍能抵抗 TRAIL 诱导的细胞凋亡。Protein O -GlcNAcylation 是一种多功能的翻译后修饰,可调节多种生物过程。最近,蛋白质O -GlcNAc 酰基化升高与癌细胞的生长和存活有关。在这项研究中,我们评估了蛋白质O -GlcNAcNA 酰化在胰腺癌 TRAIL 耐药中的作用,并鉴定了TRAIL 耐药胰腺癌细胞中较高水平的O -GlcNAcNA 酰化。随着O -GlcNAc 添加酶O -GlcNAc 转移酶 (OGT)功能的获得和丧失,我们确定增加O -GlcNAc 酰化使 TRAIL 敏感细胞对 TRA-8 诱导的细胞凋亡更具抵抗力,同时抑制O -GlcNAcylation 促进 TRA-8 诱导的 TRAIL 抗性细胞凋亡。此外,我们在体内小鼠模型中证明,OGT 敲低使 TRAIL 耐药细胞对 TRA-8 疗法敏感。机理研究揭示了 DR5 的直接O -GlcNAc 修饰,调节 TRA-8 诱导的 DR5 寡聚化。我们进一步确定 DR5 O -GlcNAc 酰化独立于 FADD,FADD 是下游死亡诱导信号传导的接头蛋白。这些研究证明了蛋白O -GlcNAc糖基化在调节胰腺癌细胞TRAIL耐药中的重要作用;并揭示了O -GlcNAcylation 对 DR5 寡聚化的贡献,从而介导 DR 诱导信号传导。


























 京公网安备 11010802027423号
京公网安备 11010802027423号