当前位置:
X-MOL 学术
›
Beilstein. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Extension of the 5-alkynyluridine side chain via C-C-bond formation in modified organometallic nucleosides using the Nicholas reaction.
Beilstein Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-01-02 , DOI: 10.3762/bjoc.16.1 Renata Kaczmarek 1 , Dariusz Korczyński 1 , James R Green 2 , Roman Dembinski 1, 3
Beilstein Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-01-02 , DOI: 10.3762/bjoc.16.1 Renata Kaczmarek 1 , Dariusz Korczyński 1 , James R Green 2 , Roman Dembinski 1, 3
Affiliation
|
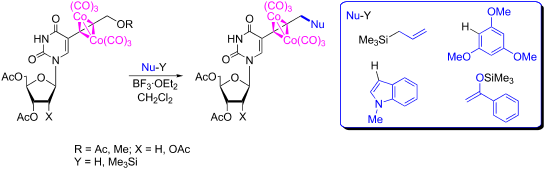
Dicobalt hexacarbonyl nucleoside complexes of propargyl ether or esters of 5-substituted uridines react with diverse C-nucleophiles. Synthetic outcomes confirmed that the Nicholas reaction can be carried out in a nucleoside presence, leading to a divergent synthesis of novel metallo-nucleosides enriched with alkene, arene, arylketo, and heterocyclic functions, in the deoxy and ribo series.
中文翻译:

使用Nicholas反应在修饰的有机金属核苷中通过CC键形成5-炔基尿苷侧链的延伸。
炔丙基醚或5-取代的尿苷的酯的二钴六羰基核苷络合物与各种C-亲核试剂反应。合成结果证实,尼古拉斯反应可在核苷存在下进行,从而导致脱氧和核糖系列中富含烯烃,芳烃,芳基酮和杂环功能的新型金属核苷的发散合成。
更新日期:2020-01-02
中文翻译:

使用Nicholas反应在修饰的有机金属核苷中通过CC键形成5-炔基尿苷侧链的延伸。
炔丙基醚或5-取代的尿苷的酯的二钴六羰基核苷络合物与各种C-亲核试剂反应。合成结果证实,尼古拉斯反应可在核苷存在下进行,从而导致脱氧和核糖系列中富含烯烃,芳烃,芳基酮和杂环功能的新型金属核苷的发散合成。


























 京公网安备 11010802027423号
京公网安备 11010802027423号