当前位置:
X-MOL 学术
›
J. Chem. Thermodyn.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Maraviroc in aqueous co-solvent solutions of n-propanol, ethanol, dimethyl sulfoxide and N,N-dimethylformamide: solubility determination, preferential solvation and solvent effect analysis
The Journal of Chemical Thermodynamics ( IF 2.6 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.jct.2019.106044 Wentian Li , Peilin Ji , Yuyuan Xu , Ali Farajtabar , Xinbao Li , Hongkun Zhao
The Journal of Chemical Thermodynamics ( IF 2.6 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.jct.2019.106044 Wentian Li , Peilin Ji , Yuyuan Xu , Ali Farajtabar , Xinbao Li , Hongkun Zhao
|
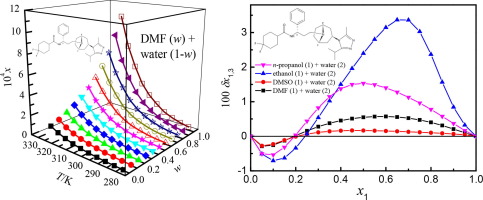
Abstract The liquid–solid equilibrium and solubility of maraviroc in co-solvent mixtures of n-propanol, ethanol, dimethyl sulfoxide (DMSO) and N,N-dimethylformamide (DMF) plus water was obtained via the saturation shake-flask technique between 278.15 K and 323.15 K under 101.2 kPa. For the four aqueous co-solvent mixtures investigated, the mole fraction solubility of maraviroc increased with the increase in the temperature and mass fraction of n-propanol, ethanol, DMSO and DMF. The maximum solubility values of maraviroc were observed in the neat co-solvents. The dependence of the maraviroc solubility on the solvent composition and temperature was mathematically correlated by means of the Jouyban-Acree model. The obtained values of relative average deviation (RAD) and root-mean-square deviation (RMSD) were no more than 4.16 × 10−2 and 49.13 × 10−6, respectively. KAT-LSER model was implemented to get quantitative information about solvent effect on the variation of solubility data. The variation in the maraviroc solubility was dependent upon the hydrogen-bond donation and cavity term in n-propanol + water and ethanol + water mixtures; and the cavity term was the dominant term in the (DMSO + water) and (DMF + water) mixtures. Quantitative study was made for the local mole fraction of ethanol (n-propanol, DMSO and DMF) and water around the maraviroc by the Inverse Kirkwood–Buff integrals method applied to the solubility data determined. Maraviroc was preferentially solvated by ethanol or n-propanol for the aqueous co-solvent mixtures of ethanol or n-propanol in intermediate and ethanol/n-propanol-rich compositions. It was conjecturable that maraviroc could act mainly as a Lewis acid in front to the ethanol or n-propanol molecules. However in the {n-propanol (1) + water (2)} mixture with compositions 0
中文翻译:

Maraviroc 在正丙醇、乙醇、二甲亚砜和 N,N-二甲基甲酰胺的共溶剂水溶液中:溶解度测定、优先溶剂化和溶剂效应分析
摘要 通过饱和摇瓶技术在 278.15 K 之间获得了马拉韦罗在正丙醇、乙醇、二甲基亚砜 (DMSO) 和 N,N-二甲基甲酰胺 (DMF) 加水的共溶剂混合物中的液固平衡和溶解度。和 101.2 kPa 下的 323.15 K。对于研究的四种水性共溶剂混合物,马拉韦罗的摩尔分数溶解度随着温度和正丙醇、乙醇、DMSO 和 DMF 质量分数的增加而增加。在纯共溶剂中观察到马拉韦罗的最大溶解度值。Maraviroc 溶解度对溶剂组成和温度的依赖性通过 Jouyban-Acree 模型在数学上相关。获得的相对平均偏差(RAD)和均方根偏差(RMSD)的值分别不超过4.16×10-2和49.13×10-6,分别。实施 KAT-LSER 模型以获取有关溶剂对溶解度数据变化影响的定量信息。maraviroc 溶解度的变化取决于正丙醇 + 水和乙醇 + 水混合物中的氢键供体和空腔项;腔项是(DMSO + 水)和(DMF + 水)混合物中的主要项。通过应用于确定的溶解度数据的逆柯克伍德-布夫积分方法,对马拉维洛克周围乙醇(正丙醇、DMSO 和 DMF)和水的局部摩尔分数进行了定量研究。对于中间体和富含乙醇/正丙醇的组合物中乙醇或正丙醇的水性共溶剂混合物,Maraviroc 优先被乙醇或正丙醇溶剂化。可以推测,maraviroc 主要充当乙醇或正丙醇分子前的路易斯酸。然而,在{正丙醇 (1) + 水 (2)} 混合物中,成分为 0
更新日期:2020-04-01
中文翻译:

Maraviroc 在正丙醇、乙醇、二甲亚砜和 N,N-二甲基甲酰胺的共溶剂水溶液中:溶解度测定、优先溶剂化和溶剂效应分析
摘要 通过饱和摇瓶技术在 278.15 K 之间获得了马拉韦罗在正丙醇、乙醇、二甲基亚砜 (DMSO) 和 N,N-二甲基甲酰胺 (DMF) 加水的共溶剂混合物中的液固平衡和溶解度。和 101.2 kPa 下的 323.15 K。对于研究的四种水性共溶剂混合物,马拉韦罗的摩尔分数溶解度随着温度和正丙醇、乙醇、DMSO 和 DMF 质量分数的增加而增加。在纯共溶剂中观察到马拉韦罗的最大溶解度值。Maraviroc 溶解度对溶剂组成和温度的依赖性通过 Jouyban-Acree 模型在数学上相关。获得的相对平均偏差(RAD)和均方根偏差(RMSD)的值分别不超过4.16×10-2和49.13×10-6,分别。实施 KAT-LSER 模型以获取有关溶剂对溶解度数据变化影响的定量信息。maraviroc 溶解度的变化取决于正丙醇 + 水和乙醇 + 水混合物中的氢键供体和空腔项;腔项是(DMSO + 水)和(DMF + 水)混合物中的主要项。通过应用于确定的溶解度数据的逆柯克伍德-布夫积分方法,对马拉维洛克周围乙醇(正丙醇、DMSO 和 DMF)和水的局部摩尔分数进行了定量研究。对于中间体和富含乙醇/正丙醇的组合物中乙醇或正丙醇的水性共溶剂混合物,Maraviroc 优先被乙醇或正丙醇溶剂化。可以推测,maraviroc 主要充当乙醇或正丙醇分子前的路易斯酸。然而,在{正丙醇 (1) + 水 (2)} 混合物中,成分为 0


























 京公网安备 11010802027423号
京公网安备 11010802027423号