当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Design and synthesis of α-naphthoflavone chimera derivatives able to eliminate cytochrome P450 (CYP)1B1-mediated drug resistance via targeted CYP1B1 degradation.
European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2020-01-02 , DOI: 10.1016/j.ejmech.2019.112028 Li Zhou 1 , Wenming Chen 2 , Chenyang Cao 3 , Yonghui Shi 4 , Wenchong Ye 3 , Jiliang Hu 3 , LingLi Wang 3 , Wen Zhou 3
European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2020-01-02 , DOI: 10.1016/j.ejmech.2019.112028 Li Zhou 1 , Wenming Chen 2 , Chenyang Cao 3 , Yonghui Shi 4 , Wenchong Ye 3 , Jiliang Hu 3 , LingLi Wang 3 , Wen Zhou 3
Affiliation
|
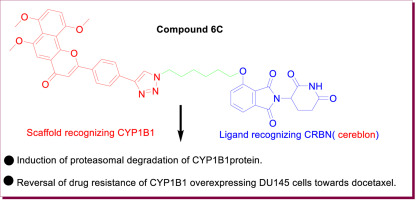
Extrahepatic cytochrome P450 1B1 (CYP1B1), which is highly expressed in various tumors, is an attractive and potential target for cancer prevention, therapy, and reversal of drug resistance. CYP1B1 inhibition is the current predominant therapeutic paradigm to treating CYP1B1-mediated malignancy, but therapeutic effect has little success. Herein, we reported CYP1B1 degradation in place of CYP1B1 inhibition for reversing drug resistance toward docetaxel in CYP1B1-overexpressing prostate cancer cell line DU145 using a PROTAC strategy. Replacing chlorine atom of a CYP1B1 selective inhibitor we found previously with ethynyl, we got the resulting α-naphthoflavone derivative 5 which kept strong inhibition against CYP1B1 (IC50 = 0.4 ± 0.2 nM) and high selectivity. Coupling of 5 with thalidomide derivatives of varying chain lengths afforded conjugates 6A-Dvia click reaction. In vitro cell-based assay indicated that 6C was more effective in eliminating drug resistance of CYP1B1-overexpressed DU145 cells compared with other analogues. Western blotting analysis showed CYP1B1 degradation was one main reason for the reversal of drug resistance to docetaxel and the effect was obtained in a concentration-dependent manner. This work is the first attempt to overcome CYP1B1-mediated drug resistance via CYP1B1 degradation instead of CYP1B1 inhibition, which could provide a new direction toward eliminating drug resistance.
中文翻译:

设计和合成能够通过靶向的CYP1B1降解消除细胞色素P450(CYP)1B1介导的耐药性的α-萘黄酮嵌合体衍生物。
肝外细胞色素P450 1B1(CYP1B1)在各种肿瘤中高表达,是预防,治疗和逆转耐药性的诱人和潜在靶标。CYP1B1抑制是目前治疗CYP1B1介导的恶性肿瘤的主要治疗方法,但是治疗效果却很少。在这里,我们报道了CYP1B1降解代替CYP1B1抑制作用以使用PROTAC策略逆转CYP1B1过表达的前列腺癌细胞系DU145对多西紫杉醇的耐药性。用乙炔基代替先前发现的CYP1B1选择性抑制剂的氯原子,得到的α-萘黄酮衍生物5保持了对CYP1B1的强抑制作用(IC50 = 0.4±0.2 nM)和高选择性。5与不同链长的沙利度胺衍生物的偶联提供了缀合物6A-Dvia点击反应。基于体外细胞的分析表明,与其他类似物相比,6C在消除CYP1B1过表达的DU145细胞的耐药性方面更有效。Western印迹分析表明,CYP1B1降解是多西紫杉醇耐药性逆转的主要原因之一,且其作用呈浓度依赖性。这项工作是通过CYP1B1降解而不是CYP1B1抑制来克服CYP1B1介导的耐药性的首次尝试,这可能为消除耐药性提供新的方向。Western印迹分析表明,CYP1B1降解是多西紫杉醇耐药性逆转的主要原因之一,且其作用呈浓度依赖性。这项工作是通过CYP1B1降解而不是CYP1B1抑制来克服CYP1B1介导的耐药性的首次尝试,这可能为消除耐药性提供新的方向。Western印迹分析表明,CYP1B1降解是多西紫杉醇耐药性逆转的主要原因之一,且其作用呈浓度依赖性。这项工作是通过CYP1B1降解而不是CYP1B1抑制来克服CYP1B1介导的耐药性的首次尝试,这可能为消除耐药性提供了新的方向。
更新日期:2020-01-02
中文翻译:

设计和合成能够通过靶向的CYP1B1降解消除细胞色素P450(CYP)1B1介导的耐药性的α-萘黄酮嵌合体衍生物。
肝外细胞色素P450 1B1(CYP1B1)在各种肿瘤中高表达,是预防,治疗和逆转耐药性的诱人和潜在靶标。CYP1B1抑制是目前治疗CYP1B1介导的恶性肿瘤的主要治疗方法,但是治疗效果却很少。在这里,我们报道了CYP1B1降解代替CYP1B1抑制作用以使用PROTAC策略逆转CYP1B1过表达的前列腺癌细胞系DU145对多西紫杉醇的耐药性。用乙炔基代替先前发现的CYP1B1选择性抑制剂的氯原子,得到的α-萘黄酮衍生物5保持了对CYP1B1的强抑制作用(IC50 = 0.4±0.2 nM)和高选择性。5与不同链长的沙利度胺衍生物的偶联提供了缀合物6A-Dvia点击反应。基于体外细胞的分析表明,与其他类似物相比,6C在消除CYP1B1过表达的DU145细胞的耐药性方面更有效。Western印迹分析表明,CYP1B1降解是多西紫杉醇耐药性逆转的主要原因之一,且其作用呈浓度依赖性。这项工作是通过CYP1B1降解而不是CYP1B1抑制来克服CYP1B1介导的耐药性的首次尝试,这可能为消除耐药性提供新的方向。Western印迹分析表明,CYP1B1降解是多西紫杉醇耐药性逆转的主要原因之一,且其作用呈浓度依赖性。这项工作是通过CYP1B1降解而不是CYP1B1抑制来克服CYP1B1介导的耐药性的首次尝试,这可能为消除耐药性提供新的方向。Western印迹分析表明,CYP1B1降解是多西紫杉醇耐药性逆转的主要原因之一,且其作用呈浓度依赖性。这项工作是通过CYP1B1降解而不是CYP1B1抑制来克服CYP1B1介导的耐药性的首次尝试,这可能为消除耐药性提供了新的方向。


























 京公网安备 11010802027423号
京公网安备 11010802027423号