当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Self-assembly of amphiphilic aryl-squaramides in water driven by dipolar π-π interactions.
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-01-08 , DOI: 10.1039/c9ob02085c Sergi Bujosa 1 , Eduardo Castellanos 1 , Antonio Frontera 1 , Carmen Rotger 1 , Antonio Costa 1 , Bartolome Soberats 1
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-01-08 , DOI: 10.1039/c9ob02085c Sergi Bujosa 1 , Eduardo Castellanos 1 , Antonio Frontera 1 , Carmen Rotger 1 , Antonio Costa 1 , Bartolome Soberats 1
Affiliation
|
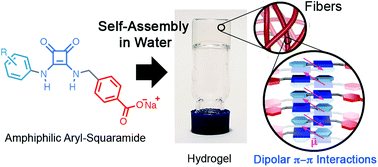
Squaramides are versatile compounds with a great capacity to interact via non-covalent interactions and therefore of interest for the development of supramolecular systems and functional materials. In the present work, a new series of aryl-squaramide amphiphiles (1-5) were prepared to form supramolecular polymers in water. Interestingly, only compounds 1 and 2 that contain electron-deficient aryl groups are capable of forming hydrogels (∼10-2 M) upon treatment with a base (NaOH or PBS). The aggregation behaviour of 1 and 2 was studied by static light scattering, UV-Vis, 1H NMR, FT-IR, and atomic force microscopy, and it was found that these compounds aggregate forming well-defined 1D nanofibers below the critical gelation concentration (<10-3 M). Moreover, the combination of these experiments with 1D and 2D NMR studies and theoretical calculations revealed that 1 and 2 self-assemble via an unprecedented interaction motif showing dipolar π-π interactions between the squaramide rings and the 4-nitrophenyl or 3,5-bis(trifluoromethyl)phenyl rings of 1 and 2, respectively. Such kinds of assemblies are stabilized by the compensation of the dipole moments of the stacked molecules. This interaction mode contrasts with those typically driving squaramide-based assemblies based on either hydrogen bonds or antiparallel stacking. We believe that this interaction motif is of interest for the design and development of new squaramide nanomaterials with free hydrogen bonding groups, which might be useful in drug delivery applications.
中文翻译:

两极π-π相互作用驱动水中的两亲芳基-方酸酰胺自组装。
方酸酰胺是通用化合物,具有通过非共价键相互作用的强大能力,因此对于超分子系统和功能材料的开发具有重要意义。在目前的工作中,制备了一系列新的芳基-方酰胺两亲物(1-5)以在水中形成超分子聚合物。有趣的是,只有含有电子不足的芳基的化合物1和2在用碱(NaOH或PBS)处理后才能形成水凝胶(〜10-2 M)。通过静态光散射,UV-Vis,1H NMR,FT-IR和原子力显微镜研究了1和2的聚集行为,发现这些化合物聚集形成低于临界胶凝浓度的清晰1D纳米纤维。 <10-3 M)。而且,这些实验与1D和2D NMR研究以及理论计算的结合表明,1和2通过空前的相互作用基序自组装,显示了方酰胺环与4-硝基苯基或3,5-双(三氟甲基)之间的偶极π-π相互作用)分别为1和2的苯环。通过补偿堆叠分子的偶极矩,可以稳定此类组件。这种相互作用模式与通常基于氢键或反平行堆积驱动基于方胺的组装的那些相反。我们认为,这种相互作用的主题是设计和开发具有自由氢键基团的新型方酸酰胺纳米材料的兴趣,这可能在药物递送应用中很有用。
更新日期:2020-02-13
中文翻译:

两极π-π相互作用驱动水中的两亲芳基-方酸酰胺自组装。
方酸酰胺是通用化合物,具有通过非共价键相互作用的强大能力,因此对于超分子系统和功能材料的开发具有重要意义。在目前的工作中,制备了一系列新的芳基-方酰胺两亲物(1-5)以在水中形成超分子聚合物。有趣的是,只有含有电子不足的芳基的化合物1和2在用碱(NaOH或PBS)处理后才能形成水凝胶(〜10-2 M)。通过静态光散射,UV-Vis,1H NMR,FT-IR和原子力显微镜研究了1和2的聚集行为,发现这些化合物聚集形成低于临界胶凝浓度的清晰1D纳米纤维。 <10-3 M)。而且,这些实验与1D和2D NMR研究以及理论计算的结合表明,1和2通过空前的相互作用基序自组装,显示了方酰胺环与4-硝基苯基或3,5-双(三氟甲基)之间的偶极π-π相互作用)分别为1和2的苯环。通过补偿堆叠分子的偶极矩,可以稳定此类组件。这种相互作用模式与通常基于氢键或反平行堆积驱动基于方胺的组装的那些相反。我们认为,这种相互作用的主题是设计和开发具有自由氢键基团的新型方酸酰胺纳米材料的兴趣,这可能在药物递送应用中很有用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号