当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enhanced Activity of the Cellulase Enzyme β-Glucosidase upon Addition of an Azobenzene-Based Surfactant
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2020-01-16 , DOI: 10.1021/acssuschemeng.9b05240 Zumra Peksaglam Seidel 1 , C. Ted Lee 1
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2020-01-16 , DOI: 10.1021/acssuschemeng.9b05240 Zumra Peksaglam Seidel 1 , C. Ted Lee 1
Affiliation
|
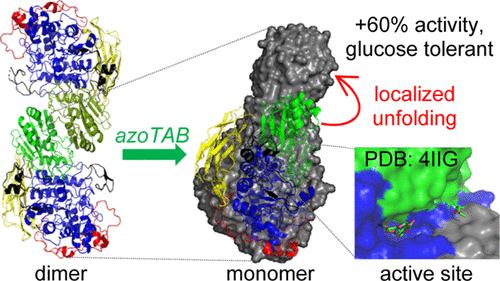
β-Glucosidases catalyze the hydrolysis of cellobiose to glucose, which is often the rate-limiting step in the conversion of cellulose into fermentable sugars during bioethanol production. Thus, the structure and function of β-glucosidase from Aspergillus niger were examined in response to a photoresponsive azobenzene-based surfactant (4-ethyl-4′(trimethylamino-butoxy)azobenzene bromide, azoTAB) as a means to enhance the enzyme activity. Light and neutron scattering data indicate that pure β-glucosidase exists as dimers or higher aggregates in solution that are progressively converted to monomers with an increasing azoTAB concentration. This transition is accompanied by a 60% increase in catalytic activity. In contrast, the enzyme is simply deactivated in the presence of conventional straight-chain hydrocarbon surfactants. Shape-reconstructed images obtained from SANS data demonstrate that azoTAB causes selective unfolding in the α/β sandwich domain that comprises the crystallographic dimer interface, consistent with the observed transition to monomers. Furthermore, this domain forms one side of a long cleft that begins at the active site and facilitates the nonproductive binding of substrate or longer oligosaccharides, which at times can block the active site. Indeed, kinetic data indicate that the azoTAB-induced increase in β-glucosidase activity is a result of diminished substrate inhibition, thus providing a unique means of obtaining glucose-tolerant β-glucosidases.
中文翻译:

添加基于偶氮苯的表面活性剂后,纤维素酶β-葡萄糖苷酶的活性增强
β-葡萄糖苷酶催化纤维二糖水解为葡萄糖,这通常是生物乙醇生产过程中纤维素转化为可发酵糖的限速步骤。因此,黑曲霉的β-葡萄糖苷酶的结构和功能针对光响应性基于偶氮苯的表面活性剂(4-乙基-4'(三甲基氨基丁氧基)偶氮苯溴化物,azoTAB)进行了检测,以增强酶的活性。光和中子散射数据表明,纯的β-葡萄糖苷酶以二聚体或更高的聚集体形式存在于溶液中,并随着azoTAB浓度的增加而逐渐转化为单体。这种转变伴随着催化活性增加了60%。相反,在常规的直链烃表面活性剂的存在下,该酶简单地失活。从SANS数据获得的形状重建图像表明azoTAB导致在包含结晶二聚体界面的α/β夹心结构域中选择性展开,这与观察到的向单体的过渡一致。此外,该结构域形成了一个长裂缝的一侧,从活性位点开始,促进了底物或更长寡糖的非生产性结合,有时会阻塞活性位点。实际上,动力学数据表明,偶氮TAB诱导的β-葡萄糖苷酶活性增加是底物抑制作用减弱的结果,因此提供了获得耐葡萄糖的β-葡萄糖苷酶的独特方法。
更新日期:2020-01-17
中文翻译:

添加基于偶氮苯的表面活性剂后,纤维素酶β-葡萄糖苷酶的活性增强
β-葡萄糖苷酶催化纤维二糖水解为葡萄糖,这通常是生物乙醇生产过程中纤维素转化为可发酵糖的限速步骤。因此,黑曲霉的β-葡萄糖苷酶的结构和功能针对光响应性基于偶氮苯的表面活性剂(4-乙基-4'(三甲基氨基丁氧基)偶氮苯溴化物,azoTAB)进行了检测,以增强酶的活性。光和中子散射数据表明,纯的β-葡萄糖苷酶以二聚体或更高的聚集体形式存在于溶液中,并随着azoTAB浓度的增加而逐渐转化为单体。这种转变伴随着催化活性增加了60%。相反,在常规的直链烃表面活性剂的存在下,该酶简单地失活。从SANS数据获得的形状重建图像表明azoTAB导致在包含结晶二聚体界面的α/β夹心结构域中选择性展开,这与观察到的向单体的过渡一致。此外,该结构域形成了一个长裂缝的一侧,从活性位点开始,促进了底物或更长寡糖的非生产性结合,有时会阻塞活性位点。实际上,动力学数据表明,偶氮TAB诱导的β-葡萄糖苷酶活性增加是底物抑制作用减弱的结果,因此提供了获得耐葡萄糖的β-葡萄糖苷酶的独特方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号