当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Systematic Investigation of Lipophilicity Modulation by Aliphatic Fluorination Motifs.
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2020-01-17 , DOI: 10.1021/acs.jmedchem.9b01172 Benjamin Jeffries 1 , Zhong Wang 1 , Hannah R Felstead 1 , Jean-Yves Le Questel 2 , James S Scott 3 , Elisabetta Chiarparin 3 , Jérôme Graton 2 , Bruno Linclau 1
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2020-01-17 , DOI: 10.1021/acs.jmedchem.9b01172 Benjamin Jeffries 1 , Zhong Wang 1 , Hannah R Felstead 1 , Jean-Yves Le Questel 2 , James S Scott 3 , Elisabetta Chiarparin 3 , Jérôme Graton 2 , Bruno Linclau 1
Affiliation
|
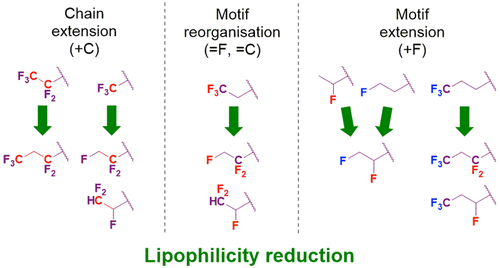
Optimization of compound lipophilicity is a key aspect of drug discovery. The aim of this work was to compare the lipophilicity modulations induced by 16 distinct known and novel fluoroalkyl motifs on three parent models. Fifty fluorinated compounds, with 28 novel experimental aliphatic log P values, are involved in discussing various lipophilicity trends. As well as confirming known trends, a number of novel lipophilicity-reducing motifs are introduced. Tactics to reduce lipophilicity are discussed, such as "motif extensions" and "motif rearrangements", including with concomitant extension of the carbon chain, as well as one- and two-fluorine 'deletions' within perfluoroalkyl groups. Quantum chemical log P calculations (SMD-MN15) based on solvent-dependent three-dimensional (3D) conformational analysis gave excellent correlations with experimental values, superior to Clog P predictions based on 2D structural motifs. The availability of a systematic collection of data based on a small number of parent molecules illustrates the relative lipophilicity modulations of aliphatic fluorination motifs.
中文翻译:

脂肪族氟化基团对亲脂性调节的系统研究。
化合物亲脂性的优化是药物发现的关键方面。这项工作的目的是比较在三个父模型上由16个不同的已知和新颖的氟烷基基序诱导的亲脂性调节。五十种具有28个新的实验性脂肪族log P值的氟化化合物参与了各种亲脂性趋势的讨论。除了确认已知趋势外,还引入了许多新颖的降低亲脂性的基序。讨论了降低亲脂性的策略,例如“基序延伸”和“基序重排”,包括碳链的同时延伸,以及全氟烷基内的一个和两个氟“缺失”。基于溶剂依赖性三维(3D)构象分析的量子化学log P计算(SMD-MN15)与实验值具有出色的相关性,优于基于2D结构基序的Clog P预测。基于少量母体分子的系统数据收集的可用性说明了脂肪族氟化基序的相对亲脂性调节。
更新日期:2020-01-17
中文翻译:

脂肪族氟化基团对亲脂性调节的系统研究。
化合物亲脂性的优化是药物发现的关键方面。这项工作的目的是比较在三个父模型上由16个不同的已知和新颖的氟烷基基序诱导的亲脂性调节。五十种具有28个新的实验性脂肪族log P值的氟化化合物参与了各种亲脂性趋势的讨论。除了确认已知趋势外,还引入了许多新颖的降低亲脂性的基序。讨论了降低亲脂性的策略,例如“基序延伸”和“基序重排”,包括碳链的同时延伸,以及全氟烷基内的一个和两个氟“缺失”。基于溶剂依赖性三维(3D)构象分析的量子化学log P计算(SMD-MN15)与实验值具有出色的相关性,优于基于2D结构基序的Clog P预测。基于少量母体分子的系统数据收集的可用性说明了脂肪族氟化基序的相对亲脂性调节。



























 京公网安备 11010802027423号
京公网安备 11010802027423号