当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cooperative Catalysis for the Highly Diastereo- and Enantioselective [4+3]-Cycloannulation of ortho-Quinone Methides and Carbonyl Ylides.
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-01-02 , DOI: 10.1002/anie.201913603 Arun Suneja 1 , Henning Jakob Loui 1 , Christoph Schneider 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-01-02 , DOI: 10.1002/anie.201913603 Arun Suneja 1 , Henning Jakob Loui 1 , Christoph Schneider 1
Affiliation
|
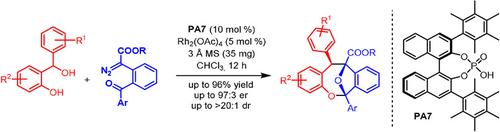
We describe herein a highly diastereo- and enantioselective [4+3]-cycloannulation of ortho-quinone methides and carbonyl ylides to furnish functionalized oxa-bridged dibenzooxacines with excellent yields and stereoselectivity in a single synthetic step. The combination of rhodium and chiral phosphoric acid catalysis working in concert to generate both transient intermediates in situ provides direct access to complex bicyclic products with two quaternary and one tertiary stereogenic centers. The products may be further functionalized into valuable and enantiomerically highly enriched building blocks.
中文翻译:

邻醌甲基化物和羰基叶立德的高度非对映和对映选择性[4+3]-环化的协同催化。
我们在此描述了邻醌甲基化物和羰基叶立德的高度非对映和对映选择性[4+3]-环化,以在单一合成步骤中提供具有优异产率和立体选择性的官能化氧杂桥联二苯并恶辛。铑和手性磷酸催化的组合协同作用,原位产生两种瞬时中间体,提供了直接获得具有两个四级和一个三级立体中心的复杂双环产物的途径。该产品可以进一步功能化为有价值且对映体高度丰富的构建模块。
更新日期:2020-01-23
中文翻译:

邻醌甲基化物和羰基叶立德的高度非对映和对映选择性[4+3]-环化的协同催化。
我们在此描述了邻醌甲基化物和羰基叶立德的高度非对映和对映选择性[4+3]-环化,以在单一合成步骤中提供具有优异产率和立体选择性的官能化氧杂桥联二苯并恶辛。铑和手性磷酸催化的组合协同作用,原位产生两种瞬时中间体,提供了直接获得具有两个四级和一个三级立体中心的复杂双环产物的途径。该产品可以进一步功能化为有价值且对映体高度丰富的构建模块。



























 京公网安备 11010802027423号
京公网安备 11010802027423号