Science Bulletin ( IF 18.9 ) Pub Date : 2019-12-31 , DOI: 10.1016/j.scib.2019.12.023 Jian Luo 1 , Guo-Shu Chen 1 , Shu-Jie Chen 1 , Yun-Lin Liu 1
|
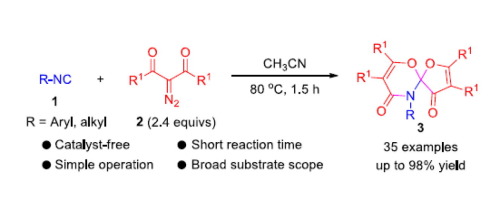
An expedient and economic approach for constructing O,O,N-spiro compounds consisting of both a 1,3-oxazine and a furan ring through a catalyst-free formal [4+1]/[4+2] cycloaddition cascade sequence of isocyanides with two molecules of acylketene formed in situ through thermal-induced Wolff rearrangement of 2-diazo-1,3-diketones was developed. The reaction displayed good functional group tolerance and was compatible with different isocyanides and 2-diazo-1,3-diketones. Furthermore, preliminary asymmetric attempts of this reaction are made by utilizing optically pure isocyanides as inputs, and moderate diastereomeric induction was observed.
中文翻译:

异氰化物的无催化剂形式 [4+1]/[4+2] 环化级联序列与 2-重氮-1,3-二酮的热诱导沃尔夫重排原位形成的两个酰基乙烯酮分子
一种通过异氰化物的无催化剂形式 [4+1]/[4+2] 环加成级联序列构建由 1,3-恶嗪和呋喃环组成的O , O , N -螺环化合物的简便且经济的方法开发了通过 2-重氮-1,3-二酮的热诱导沃尔夫重排原位形成的两个酰基乙烯酮分子。该反应显示出良好的官能团耐受性,并且与不同的异氰化物和 2-重氮-1,3-二酮相容。此外,该反应的初步不对称尝试是通过使用光学纯异氰化物作为输入进行的,并且观察到适度的非对映体诱导。



























 京公网安备 11010802027423号
京公网安备 11010802027423号