当前位置:
X-MOL 学术
›
Arab. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hypervalent iodine(III) catalyzed rapid and efficient access to benzimidazoles, benzothiazoles and quinoxalines: Biological evaluation of some new benzimidazole-imidazo[1,2- a ]pyridine conjugates
Arabian Journal of Chemistry ( IF 6 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.arabjc.2017.02.007 P.V. Sri Ramya , Srinivas Angapelly , Routhu Sunitha Rani , Chander Singh Digwal , C. Ganesh Kumar , Bathini Nagendra Babu , Lalita Guntuku , Ahmed Kamal
Arabian Journal of Chemistry ( IF 6 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.arabjc.2017.02.007 P.V. Sri Ramya , Srinivas Angapelly , Routhu Sunitha Rani , Chander Singh Digwal , C. Ganesh Kumar , Bathini Nagendra Babu , Lalita Guntuku , Ahmed Kamal
|
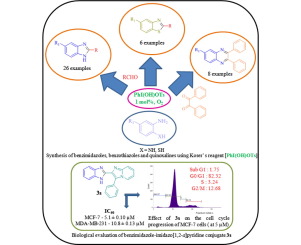
Abstract A rapid, simple and highly efficient method for the synthesis of a variety of 2-aryl-benzimidazoles, 2-aryl-benzothiazoles and quinoxalines has been developed using Koser’s reagent [PhI(OH)OTs] as catalyst. The present work highlights the potential of Koser's reagent ([PhI(OH)OTs]) for the synthesis of benzimidazoles, benzothiazoles and quinoxalines, etc. Short reaction time, high yields, importantly low catalyst loading, broad substrate scope and scalability are the salient features of this methodology. Particularly, this method has been employed successfully to synthesize highly structured indole-benzimidazole and quinoxaline-6-carboxamide derivatives as well as biologically important benzimidazole-imidazo[1,2-a]pyridine conjugates in moderate to good yields. These remarkable features make the present methodology a valid contribution to the existing precedents for the synthesis of benzimidazoles, etc. In the MTT assay, benzimidazole-imidazo[1,2-a]pyridine conjugates 3s, 3t and 3v were found to be active on MCF-7 (IC50 values of 5.10 ± 0.10, 8.23 ± 0.02, and 10.75 ± 0.03 µM, respectively) and MDA-MB-231 cell lines (IC50 values of 10.83 ± 0.13, 7.68 ± 0.05, and 7.87 ± 0.24 µM, respectively). Flow-cytometry analysis revealed that the treatment of MCF-7 cells with compound 3s showed moderate effect on the progression of G0/G1 phase of the cell cycle.
中文翻译:

高价碘 (III) 催化快速有效地获得苯并咪唑、苯并噻唑和喹喔啉:一些新型苯并咪唑-咪唑并[1,2-a] 吡啶偶联物的生物学评价
摘要 以Koser试剂[PhI(OH)OTs]为催化剂,开发了一种快速、简单、高效的合成多种2-芳基-苯并咪唑、2-芳基-苯并噻唑和喹喔啉的方法。目前的工作突出了 Koser 试剂 ([PhI(OH)OTs]) 在合成苯并咪唑、苯并噻唑和喹喔啉等方面的潜力。反应时间短、产率高、催化剂负载量低、底物范围广和可扩展性是突出的这种方法论的特点。特别是,该方法已成功用于合成高度结构化的吲哚-苯并咪唑和喹喔啉-6-甲酰胺衍生物以及生物学上重要的苯并咪唑-咪唑并 [1,2-a] 吡啶偶联物,收率中等至良好。这些显着的特征使本方法对合成苯并咪唑等的现有先例做出有效贡献。 在 MTT 测定中,发现苯并咪唑-咪唑并 [1,2-a] 吡啶缀合物 3s、3t 和 3v 对MCF-7(IC50 值分别为 5.10 ± 0.10、8.23 ± 0.02 和 10.75 ± 0.03 µM)和 MDA-MB-231 细胞系(IC50 值分别为 10.83 ± 0.13、7.68 ± 0.05 和 0.05 ± 7 M )。流式细胞术分析表明,用化合物 3s 处理 MCF-7 细胞对细胞周期的 G0/G1 期进展显示出中等影响。分别)和 MDA-MB-231 细胞系(IC50 值分别为 10.83 ± 0.13、7.68 ± 0.05 和 7.87 ± 0.24 µM)。流式细胞术分析表明,用化合物 3s 处理 MCF-7 细胞对细胞周期的 G0/G1 期进展显示出中等影响。分别)和 MDA-MB-231 细胞系(IC50 值分别为 10.83 ± 0.13、7.68 ± 0.05 和 7.87 ± 0.24 µM)。流式细胞术分析表明,用化合物 3s 处理 MCF-7 细胞对细胞周期的 G0/G1 期进展显示出中等影响。
更新日期:2020-01-01
中文翻译:

高价碘 (III) 催化快速有效地获得苯并咪唑、苯并噻唑和喹喔啉:一些新型苯并咪唑-咪唑并[1,2-a] 吡啶偶联物的生物学评价
摘要 以Koser试剂[PhI(OH)OTs]为催化剂,开发了一种快速、简单、高效的合成多种2-芳基-苯并咪唑、2-芳基-苯并噻唑和喹喔啉的方法。目前的工作突出了 Koser 试剂 ([PhI(OH)OTs]) 在合成苯并咪唑、苯并噻唑和喹喔啉等方面的潜力。反应时间短、产率高、催化剂负载量低、底物范围广和可扩展性是突出的这种方法论的特点。特别是,该方法已成功用于合成高度结构化的吲哚-苯并咪唑和喹喔啉-6-甲酰胺衍生物以及生物学上重要的苯并咪唑-咪唑并 [1,2-a] 吡啶偶联物,收率中等至良好。这些显着的特征使本方法对合成苯并咪唑等的现有先例做出有效贡献。 在 MTT 测定中,发现苯并咪唑-咪唑并 [1,2-a] 吡啶缀合物 3s、3t 和 3v 对MCF-7(IC50 值分别为 5.10 ± 0.10、8.23 ± 0.02 和 10.75 ± 0.03 µM)和 MDA-MB-231 细胞系(IC50 值分别为 10.83 ± 0.13、7.68 ± 0.05 和 0.05 ± 7 M )。流式细胞术分析表明,用化合物 3s 处理 MCF-7 细胞对细胞周期的 G0/G1 期进展显示出中等影响。分别)和 MDA-MB-231 细胞系(IC50 值分别为 10.83 ± 0.13、7.68 ± 0.05 和 7.87 ± 0.24 µM)。流式细胞术分析表明,用化合物 3s 处理 MCF-7 细胞对细胞周期的 G0/G1 期进展显示出中等影响。分别)和 MDA-MB-231 细胞系(IC50 值分别为 10.83 ± 0.13、7.68 ± 0.05 和 7.87 ± 0.24 µM)。流式细胞术分析表明,用化合物 3s 处理 MCF-7 细胞对细胞周期的 G0/G1 期进展显示出中等影响。


























 京公网安备 11010802027423号
京公网安备 11010802027423号