当前位置:
X-MOL 学术
›
Arab. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis, antimicrobial activity and docking study of some novel 4-(4,4-dimethyl-2,6-dioxocyclohexylidene)methylamino derivatives carrying biologically active sulfonamide moiety
Arabian Journal of Chemistry ( IF 6 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.arabjc.2017.05.022 Mostafa M. Ghorab , Aiten M. Soliman , Mansour S. Alsaid , Ahmed A. Askar
Arabian Journal of Chemistry ( IF 6 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.arabjc.2017.05.022 Mostafa M. Ghorab , Aiten M. Soliman , Mansour S. Alsaid , Ahmed A. Askar
|
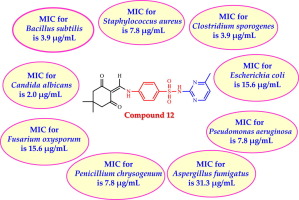
Abstract A new series of 4-((4,4-dimethyl-2,6-dioxocyclohexylidene)methylamino)- N -(substituted)benzenesulfonamide 3 – 17 , monosubstituted 2-((4-((4-aminophenyl)sulfonyl)phenyl)amino)methylene 18 , and its disubstituted derivative 19 were synthesized from the starting material 2-((dimethylamino)methylene)-5,5-dimethylcyclohexane-1,3-dione 2 . The crystal structures of compounds 2 , 7 and 13 were reported by us through X-ray crystallography. All the prepared compounds were evaluated for their antibacterial activity against Gram-positive bacteria ( Staphylococcus aureus , Bacillus subtilis , Clostridium sporogenes ), Gram-negative bacteria ( Pseudomonas aeruginosa , Escherichia coli ), and antifungal activity against Aspergillus fumigatus , Penicillium chrysogenum , Fusarium oxysporum , Candida albicans. The synthesized compounds displayed interesting antimicrobial activity. Compounds 4 and 12 were the most potent in this study and displayed higher activity compared to the reference drugs, with MIC value of 3.9–31.3 μg/mL against a panel of Gram-positive, Gram-negative bacteria and fungi. Molecular modeling was performed inside the active site of dihydropteroate synthase. The synthesized compounds showed similar orientation and binding interactions to that of the co-crystallized ligand inside the binding pocket.
中文翻译:

一些带有生物活性磺酰胺部分的新型4-(4,4-二甲基-2,6-二氧代环己叉)甲氨基衍生物的合成、抗菌活性和对接研究
摘要 4-((4,4-二甲基-2,6-二氧代环己基)甲氨基)-N-(取代)苯磺酰胺3-17、单取代2-((4-((4-氨基苯基)磺酰基)苯基)氨基)亚甲基 18 及其二取代衍生物 19 由起始原料 2-((二甲基氨基) 亚甲基)-5,5-二甲基环己烷-1,3-二酮 2 合成。我们通过X射线晶体学报道了化合物2、7和13的晶体结构。评价了制备的所有化合物对革兰氏阳性菌(金黄色葡萄球菌、枯草芽孢杆菌、产孢梭菌)、革兰氏阴性菌(铜绿假单胞菌、大肠杆菌)的抗菌活性,以及对烟曲霉、青霉、镰刀菌的抗真菌活性, 白色念珠菌。合成的化合物显示出有趣的抗菌活性。化合物 4 和 12 在本研究中最有效,与参考药物相比显示出更高的活性,对一组革兰氏阳性、革兰氏阴性细菌和真菌的 MIC 值为 3.9-31.3 μg/mL。在二氢蝶酸合酶的活性位点内进行分子建模。合成的化合物显示出与结合口袋内的共结晶配体相似的取向和结合相互作用。
更新日期:2020-01-01
中文翻译:

一些带有生物活性磺酰胺部分的新型4-(4,4-二甲基-2,6-二氧代环己叉)甲氨基衍生物的合成、抗菌活性和对接研究
摘要 4-((4,4-二甲基-2,6-二氧代环己基)甲氨基)-N-(取代)苯磺酰胺3-17、单取代2-((4-((4-氨基苯基)磺酰基)苯基)氨基)亚甲基 18 及其二取代衍生物 19 由起始原料 2-((二甲基氨基) 亚甲基)-5,5-二甲基环己烷-1,3-二酮 2 合成。我们通过X射线晶体学报道了化合物2、7和13的晶体结构。评价了制备的所有化合物对革兰氏阳性菌(金黄色葡萄球菌、枯草芽孢杆菌、产孢梭菌)、革兰氏阴性菌(铜绿假单胞菌、大肠杆菌)的抗菌活性,以及对烟曲霉、青霉、镰刀菌的抗真菌活性, 白色念珠菌。合成的化合物显示出有趣的抗菌活性。化合物 4 和 12 在本研究中最有效,与参考药物相比显示出更高的活性,对一组革兰氏阳性、革兰氏阴性细菌和真菌的 MIC 值为 3.9-31.3 μg/mL。在二氢蝶酸合酶的活性位点内进行分子建模。合成的化合物显示出与结合口袋内的共结晶配体相似的取向和结合相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号