当前位置:
X-MOL 学术
›
J. Inorg. Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Formation and kinetic studies of manganese(IV)-oxo porphyrins: Oxygen atom transfer mechanism of sulfide oxidations.
Journal of Inorganic Biochemistry ( IF 3.9 ) Pub Date : 2019-12-31 , DOI: 10.1016/j.jinorgbio.2019.110986 Seth Klaine 1 , Fox Bratcher 1 , Charles M Winchester 1 , Rui Zhang 1
Journal of Inorganic Biochemistry ( IF 3.9 ) Pub Date : 2019-12-31 , DOI: 10.1016/j.jinorgbio.2019.110986 Seth Klaine 1 , Fox Bratcher 1 , Charles M Winchester 1 , Rui Zhang 1
Affiliation
|
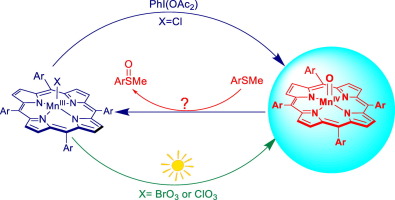
Visible light irradiation of photo-labile porphyrin-manganese(III) chlorates or bromates (2) produced manganese(IV)-oxo porphyrins [MnIV(Por)(O)] (Por = porphyrin) (3) in three porphyrin ligands. The same oxo species 3 were also formed by chemical oxidation of the corresponding manganese(III) precursors (1) with iodobenzene diacetate, i.e. PhI(OAc)2. The systems under study include 5,10,15,20-tetra(pentafluorophenyl)porphyrin‑manganese(IV)-oxo (3a), 5,10,15,20-tetra(2,6-difluorophenyl)porphyrin‑manganese(IV)-oxo (3b), and 5,10,15,20-tetramesitylporphyrin‑manganese(IV)-oxo (3c). As expected, complexes 3 reacted with thioanisoles to produce the corresponding sulfoxides and over-oxidized sulfones. The kinetics of oxygen atom transfer (OAT) reactions of these generated 3 with aryl sulfides were studied in CH3CN solutions. Second-order rate constants for sulfide oxidation reactions are comparable to those of alkene epoxidations and activated CH bond oxidations by the same oxo species 3. For a given substrate, the reactivity order for the manganese(IV)-oxo species was 3a > 3b > 3c, consistent with expectations on the basis of the electron-withdrawing capacity of the porphyrin macrocycles. Free-energy Hammett analyses gave near-linear correlations with σ values, indicating no significant positive charge developed at the sulfur during the oxidation process. The mechanistic results strongly suggest [MnIV(Por)(O)] reacts as a direct OAT agent towards sulfide substrates through a manganese(II) intermediate that was detected in this work. However, an alternative pathway that involves a disproportionation of 3 to form a higher oxidized manganese(V)-oxo species may be significant when less reactive substrates are present. The competition product studies with the Hammett correlation plot confirmed that the observed manganese(IV)-oxo species is not the true oxidant for the sulfide oxidations catalyzed by manganese(III) porphyrins with PhI(OAc)2.
中文翻译:

锰(IV)-氧卟啉的形成和动力学研究:硫化物氧化的氧原子转移机理。
在三个卟啉配体中,对光不稳定的卟啉-锰(III)的氯酸盐或溴酸盐(2)的可见光照射产生了锰(IV)-氧代卟啉[MnIV(Por)(O)](Por =卟啉)(3)。通过用碘代苯二乙酸盐,即PhI(OAc)2对相应的锰(III)前体(1)进行化学氧化,也形成了相同的羰基化合物3。所研究的系统包括5,10,15,20-四(五氟苯基)卟啉-锰(IV)-氧(3a),5,10,15,20-四(2,6-二氟苯基)卟啉-锰(IV )-氧代(3b)和5,10,15,20-四甲苯基卟啉-锰(IV)-氧代(3c)。如所预期的,配合物3与硫代苯甲醚反应以产生相应的亚砜和过氧化的砜。在CH3CN溶液中研究了这些生成的3与芳基硫化物的氧原子转移(OAT)反应动力学。硫化物氧化反应的二级速率常数与相同的羰基化合物3的烯烃环氧化和活化的CH键氧化反应的速率常数相当。对于给定的底物,锰(IV)-羰基化合物的反应顺序为3a> 3b> 3c,与基于卟啉大环的吸电子能力的预期一致。自由能Hammett分析给出了与σ值近似线性的相关性,表明在氧化过程中硫上没有显着的正电荷产生。机理结果强烈表明,[MnIV(Por)(O)]作为直接的OAT试剂通过在该工作中检测到的锰(II)中间体向硫化物底物反应。然而,当存在反应性较低的底物时,涉及3歧化以形成较高氧化锰(V)-氧代物种的替代途径可能很重要。利用Hammett相关图进行的竞争产品研究证实,观察到的锰(IV)-氧代物种不是锰(III)卟啉与PhI(OAc)2催化的硫化物氧化的真正氧化剂。
更新日期:2019-12-31
中文翻译:

锰(IV)-氧卟啉的形成和动力学研究:硫化物氧化的氧原子转移机理。
在三个卟啉配体中,对光不稳定的卟啉-锰(III)的氯酸盐或溴酸盐(2)的可见光照射产生了锰(IV)-氧代卟啉[MnIV(Por)(O)](Por =卟啉)(3)。通过用碘代苯二乙酸盐,即PhI(OAc)2对相应的锰(III)前体(1)进行化学氧化,也形成了相同的羰基化合物3。所研究的系统包括5,10,15,20-四(五氟苯基)卟啉-锰(IV)-氧(3a),5,10,15,20-四(2,6-二氟苯基)卟啉-锰(IV )-氧代(3b)和5,10,15,20-四甲苯基卟啉-锰(IV)-氧代(3c)。如所预期的,配合物3与硫代苯甲醚反应以产生相应的亚砜和过氧化的砜。在CH3CN溶液中研究了这些生成的3与芳基硫化物的氧原子转移(OAT)反应动力学。硫化物氧化反应的二级速率常数与相同的羰基化合物3的烯烃环氧化和活化的CH键氧化反应的速率常数相当。对于给定的底物,锰(IV)-羰基化合物的反应顺序为3a> 3b> 3c,与基于卟啉大环的吸电子能力的预期一致。自由能Hammett分析给出了与σ值近似线性的相关性,表明在氧化过程中硫上没有显着的正电荷产生。机理结果强烈表明,[MnIV(Por)(O)]作为直接的OAT试剂通过在该工作中检测到的锰(II)中间体向硫化物底物反应。然而,当存在反应性较低的底物时,涉及3歧化以形成较高氧化锰(V)-氧代物种的替代途径可能很重要。利用Hammett相关图进行的竞争产品研究证实,观察到的锰(IV)-氧代物种不是锰(III)卟啉与PhI(OAc)2催化的硫化物氧化的真正氧化剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号