当前位置:
X-MOL 学术
›
Fluid Phase Equilibr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Influence of polymeric excipients on the solubility of aspirin: Experimental measurement and model prediction
Fluid Phase Equilibria ( IF 2.6 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.fluid.2019.112450 Dongxu Wu , Yuanhui Ji
Fluid Phase Equilibria ( IF 2.6 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.fluid.2019.112450 Dongxu Wu , Yuanhui Ji
|
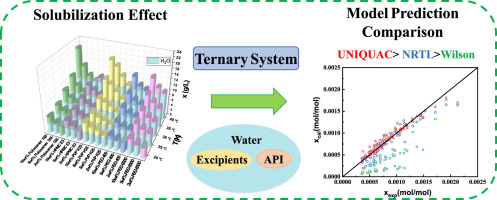
Abstract In this work, the solubility of aspirin at 293.15–313.15 K was measured in several aqueous systems where one excipient out of PEG 6000, PEG 400, PVP K25, HPMC E3 and poloxamer 188 was present. It was found that PVP K25 had the strongest solubilizing ability and the solubility enhancement of aspirin by poloxamer 188 was greatly affected by temperature. In addition, the Wilson, NRTL and UNIQUAC models were employed to predict the solubility of aspirin in various organic solvents as well as in different mixtures of polymeric excipients and water through interaction parameters obtained from experimental data. The average ARDs of Wilson, NRTL, and UNIQUAC models were 0.0300, 0.0318, 0.0305 for the binary aspirin-solvent systems, respectively, and those were 0.4214, 0.1166, 0.0861 for the ternary aspirin-polymer-water systems. UNIQUAC model among these three models was found to have excellent performance for solubility prediction of aspirin in solvents and polymer-water solutions.
中文翻译:

聚合物赋形剂对阿司匹林溶解度的影响:实验测量和模型预测
摘要 在这项工作中,阿司匹林在 293.15–313.15 K 下的溶解度在几种水性体系中进行了测量,其中存在 PEG 6000、PEG 400、PVP K25、HPMC E3 和泊洛沙姆 188 中的一种赋形剂。发现PVP K25的增溶能力最强,泊洛沙姆188对阿司匹林溶解度的增强受温度影响较大。此外,Wilson、NRTL 和 UNIQUAC 模型用于通过从实验数据获得的相互作用参数预测阿司匹林在各种有机溶剂以及聚合物赋形剂和水的不同混合物中的溶解度。Wilson、NRTL和UNIQUAC模型的平均ARDs对于二元阿司匹林-溶剂系统分别为0.0300、0.0318、0.0305,对于三元阿司匹林-聚合物-水系统分别为0.4214、0.1166、0.0861。
更新日期:2020-03-01
中文翻译:

聚合物赋形剂对阿司匹林溶解度的影响:实验测量和模型预测
摘要 在这项工作中,阿司匹林在 293.15–313.15 K 下的溶解度在几种水性体系中进行了测量,其中存在 PEG 6000、PEG 400、PVP K25、HPMC E3 和泊洛沙姆 188 中的一种赋形剂。发现PVP K25的增溶能力最强,泊洛沙姆188对阿司匹林溶解度的增强受温度影响较大。此外,Wilson、NRTL 和 UNIQUAC 模型用于通过从实验数据获得的相互作用参数预测阿司匹林在各种有机溶剂以及聚合物赋形剂和水的不同混合物中的溶解度。Wilson、NRTL和UNIQUAC模型的平均ARDs对于二元阿司匹林-溶剂系统分别为0.0300、0.0318、0.0305,对于三元阿司匹林-聚合物-水系统分别为0.4214、0.1166、0.0861。


























 京公网安备 11010802027423号
京公网安备 11010802027423号