当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Role of Local Structure on Catalytic Reactivity: Comparison of Methanol Oxidation by Aqueous Bioinorganic Enzyme Mimic (Vanadium Haloperoxidase) and Vanadia-Based Heterogeneous Catalyst (Supported VO4/SiO2)
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-01-08 , DOI: 10.1021/acscatal.9b02450 Ozgen Yalcin 1, 2 , Julie E. Molinari Erwin 1 , Duygu Gerceker 2 , Isik Onal 2 , Israel E. Wachs 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-01-08 , DOI: 10.1021/acscatal.9b02450 Ozgen Yalcin 1, 2 , Julie E. Molinari Erwin 1 , Duygu Gerceker 2 , Isik Onal 2 , Israel E. Wachs 1
Affiliation
|
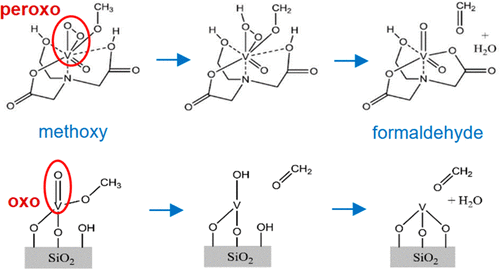
Although enzymes perform redox reactions at milder reaction conditions than heterogeneous solid catalysts, the origin of this reactivity difference still needs to be resolved. In the present study, the mechanisms and kinetics of methanol oxidation by the aqueous bioinorganic vanadium haloperoxidase (VHPO) enzyme mimic and the heterogeneous solid oxide supported VO4/SiO2 catalyst operating in the gas–solid reaction are compared in order to address the origin of the different reactivities. The molecular level chemical transformations during methanol oxidation by the aqueous VHPO enzyme mimic, possessing the oxo-peroxo O═VO2(−O—C) functionalities, and heterogeneous supported VO4/SiO2 catalysts, containing isolated vanadyl O═V(−O—Si)3 sites, were monitored with time-resolved in situ vibrational spectroscopy (Raman and IR). Both catalytic reactions proceed via the same methoxy (V–OCH3) reaction intermediate formed upon methanol chemisorption at the bridging V–O–C/V–O–Si bonds. The difference in reactivity is related to the much lower activation energy for breaking the C–H bond of the V–OCH3 intermediate, the rate-determining step, by the highly reactive vanadium peroxo VO2 sites only present for bioinorganic enzyme mimics and absent from heterogeneous catalysts under reaction conditions.
中文翻译:

局部结构在催化反应性上的作用:比较生物含水模拟酶(钒卤代过氧化物酶)和钒基非均相催化剂(负载的VO 4 / SiO 2)对甲醇的氧化
尽管酶在比异质固体催化剂更温和的反应条件下进行氧化还原反应,但仍需要解决这种反应性差异的根源。在本研究中,比较了生物无机钒水溶液卤过氧化物酶(VHPO)酶模拟物和在气固反应中运行的多相固体氧化物负载的VO 4 / SiO 2催化剂进行甲醇氧化的机理和动力学,从而解决了这一起源。不同的反应性。甲醇氧化过程中由水性VHPO酶分子水平的化学转化模拟物,具有的氧代-过氧O═VO 2(-O-C)的功能,和异构支持VO 4 /二氧化硅2用时间分辨原位振动光谱法(拉曼光谱和红外光谱)监测含有孤立的钒基O═V(-O-Si)3位的催化剂。两种催化反应均通过相同的甲氧基(V–OCH 3)反应中间体进行,该中间体是在甲醇化学吸附时在桥接的V–OC–C / V–O–Si键上形成的。反应性的差异与通过仅存在于生物无机酶模拟物中且不存在的高反应性钒过氧VO 2位点来破坏V-OCH 3中间体的C-H键(速率决定步骤)的活化能低得多有关在反应条件下由多相催化剂产生。
更新日期:2020-01-08
中文翻译:

局部结构在催化反应性上的作用:比较生物含水模拟酶(钒卤代过氧化物酶)和钒基非均相催化剂(负载的VO 4 / SiO 2)对甲醇的氧化
尽管酶在比异质固体催化剂更温和的反应条件下进行氧化还原反应,但仍需要解决这种反应性差异的根源。在本研究中,比较了生物无机钒水溶液卤过氧化物酶(VHPO)酶模拟物和在气固反应中运行的多相固体氧化物负载的VO 4 / SiO 2催化剂进行甲醇氧化的机理和动力学,从而解决了这一起源。不同的反应性。甲醇氧化过程中由水性VHPO酶分子水平的化学转化模拟物,具有的氧代-过氧O═VO 2(-O-C)的功能,和异构支持VO 4 /二氧化硅2用时间分辨原位振动光谱法(拉曼光谱和红外光谱)监测含有孤立的钒基O═V(-O-Si)3位的催化剂。两种催化反应均通过相同的甲氧基(V–OCH 3)反应中间体进行,该中间体是在甲醇化学吸附时在桥接的V–OC–C / V–O–Si键上形成的。反应性的差异与通过仅存在于生物无机酶模拟物中且不存在的高反应性钒过氧VO 2位点来破坏V-OCH 3中间体的C-H键(速率决定步骤)的活化能低得多有关在反应条件下由多相催化剂产生。



























 京公网安备 11010802027423号
京公网安备 11010802027423号