当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
NMR "Crystallography" for Uniformly (13 C, 15 N)-Labeled Oriented Membrane Proteins.
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2019-12-30 , DOI: 10.1002/anie.201915110 Emmanuel O Awosanya 1 , Joel Lapin 1 , Alexander A Nevzorov 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2019-12-30 , DOI: 10.1002/anie.201915110 Emmanuel O Awosanya 1 , Joel Lapin 1 , Alexander A Nevzorov 1
Affiliation
|
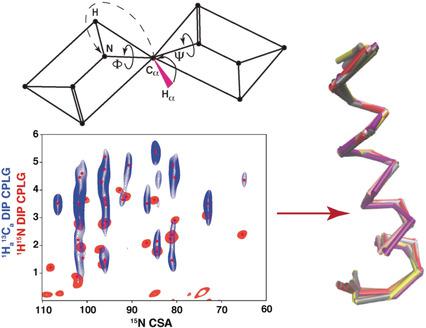
In oriented-sample (OS) solid-state NMR of membrane proteins, the angular-dependent dipolar couplings and chemical shifts provide a direct input for structure calculations. However, so far only 1 H-15 N dipolar couplings and 15 N chemical shifts have been routinely assessed in oriented 15 N-labeled samples. The main obstacle for extending this technique to membrane proteins of arbitrary topology has remained in the lack of additional experimental restraints. We have developed a new experimental triple-resonance NMR technique, which was applied to uniformly doubly (15 N, 13 C)-labeled Pf1 coat protein in magnetically aligned DMPC/DHPC bicelles. The previously inaccessible 1 Hα -13 Cα dipolar couplings have been measured, which make it possible to determine the torsion angles between the peptide planes without assuming α-helical structure a priori. The fitting of three angular restraints per peptide plane and filtering by Rosetta scoring functions has yielded a consensus α-helical transmembrane structure for Pf1 protein.
中文翻译:

均一(13 C,15 N)标记的膜蛋白的NMR“晶体学”。
在膜蛋白的定向样品(OS)固态NMR中,取决于角度的偶极偶合和化学位移为结构计算提供了直接的输入。然而,到目前为止,在定向的15 N标记样品中,常规评估仅1 H-15 N偶极偶合和15 N化学位移。缺乏额外的实验限制仍然是将该技术扩展到任意拓扑的膜蛋白的主要障碍。我们已经开发了一种新的实验性三共振NMR技术,该技术已应用于磁性排列的DMPC / DHPC双细胞中均匀加倍(15 N,13 C)标记的Pf1外壳蛋白。已经测量了以前无法接近的1Hα-13Cα偶极偶合,这使得无需先验假设α螺旋结构就可以确定肽平面之间的扭转角。
更新日期:2020-01-23
中文翻译:

均一(13 C,15 N)标记的膜蛋白的NMR“晶体学”。
在膜蛋白的定向样品(OS)固态NMR中,取决于角度的偶极偶合和化学位移为结构计算提供了直接的输入。然而,到目前为止,在定向的15 N标记样品中,常规评估仅1 H-15 N偶极偶合和15 N化学位移。缺乏额外的实验限制仍然是将该技术扩展到任意拓扑的膜蛋白的主要障碍。我们已经开发了一种新的实验性三共振NMR技术,该技术已应用于磁性排列的DMPC / DHPC双细胞中均匀加倍(15 N,13 C)标记的Pf1外壳蛋白。已经测量了以前无法接近的1Hα-13Cα偶极偶合,这使得无需先验假设α螺旋结构就可以确定肽平面之间的扭转角。


























 京公网安备 11010802027423号
京公网安备 11010802027423号