当前位置:
X-MOL 学术
›
JAMA Neurol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development and Validation of the Rasch-Built Overall Amyotrophic Lateral Sclerosis Disability Scale (ROADS).
JAMA Neurology ( IF 29.0 ) Pub Date : 2020-04-01 , DOI: 10.1001/jamaneurol.2019.4490 Christina N Fournier 1, 2 , Richard Bedlack 3 , Colin Quinn 4 , James Russell 5 , Diane Beckwith 2 , Kathleen H Kaminski 1 , William Tyor 1, 2 , Vicki Hertzberg 2 , Virginia James 2 , Meraida Polak 2 , Jonathan D Glass 2
JAMA Neurology ( IF 29.0 ) Pub Date : 2020-04-01 , DOI: 10.1001/jamaneurol.2019.4490 Christina N Fournier 1, 2 , Richard Bedlack 3 , Colin Quinn 4 , James Russell 5 , Diane Beckwith 2 , Kathleen H Kaminski 1 , William Tyor 1, 2 , Vicki Hertzberg 2 , Virginia James 2 , Meraida Polak 2 , Jonathan D Glass 2
Affiliation
|
Importance
A new outcome measure for overall disability level with improved responsiveness is needed for amyotrophic lateral sclerosis (ALS) clinical trials.
Objective
To describe the creation and development of a new self-reported ALS disability scale with improved item targeting and psychometric properties that used a mathematically rigorous Rasch methodology.
Design, Setting, and Participants
A preliminary ALS disability questionnaire with 119 questions was created based on literature review, clinical judgement of an expert panel, and patient input. Patients with ALS were recruited from January 2017 to June 2019 from the Emory University and Atlanta VA Medical Center ALS clinics, both in Atlanta, Georgia, during regularly scheduled clinic appointments to complete the draft questionnaire and standard ALS outcome measures. All consecutive patients seen at the Emory University and Atlanta VA Medical Center ALS clinics during the recruitment period with a diagnosis of ALS who were able to provide informed consent were invited to participate in the study. Rasch analyses were performed, and items were systematically removed based on missing data, model fit, disordered thresholds, item bias, and clinical judgment. A total of 509 patients with ALS were seen at the 2 sites during the recruitment period, and 264 patients provided informed consent.
Interventions
Participants completed the draft Rasch questionnaire and the revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R).
Main Outcomes and Measures
Rasch analyses and standard scale metrics were performed to create the new scale, and Rasch analyses were performed on the ALSFRS-R for comparison.
Results
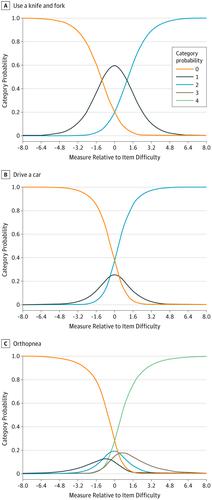
Overall, 243 participants with ALS completed the draft questionnaire, and 230 participants were included for Rasch analyses. The mean (SD) age for study participants was 61.9 (11.1) years, 146 (60.1%) were men, and site of onset was 23.0% bulbar (n = 56), 36.2% upper extremity (n = 88), and 39.5% lower extremity (n = 96). A 28-question Rasch-Built Overall ALS Disability Scale (ROADS) was constructed with each item scored 0, 1, or 2. The ROADS fulfilled Rasch model requirements, demonstrated improved item targeting compared with the ALSFRS-R, and had test-retest reliability of 0.97. Individual question fit statistics demonstrated infit values from 0.68 to 1.37 and outfit values from 0.66 to 1.43. The difference between the empirical variance explained by the measures and the modeled variance was 0.1%. The ALSFRS-R violated Rasch model expectations and demonstrated disordered thresholds for 9 of 12 questions; 13 of 48 answer choices on the ALSFRS-R were never the most probable answer choice for any overall disability level.
Conclusions and Relevance
In this study, the 28-question, self-reported ROADS, which is linearly weighted, had improved item targeting compared with the ALSFRS-R, had high test-retest reliability, and was validated. ROADS may serve as a valuable and easily accessible outcome measure for use in ALS trials and in the clinic with improved responsiveness compared with the ALSFRS-R.
中文翻译:

Rasch建立的整体肌萎缩性侧索硬化病残量表(ROADS)的开发和验证。
重要性肌萎缩性侧索硬化症(ALS)临床试验需要一种具有改善反应性的整体残疾水平的新结局指标。目的描述一种新的自我报告的ALS残疾量表的创建和开发,该量表使用数学严格的Rasch方法改进了项目的针对性和心理计量学特性。设计,设置和参与者基于文献回顾,专家组的临床判断和患者意见,创建了一个包含119个问题的ALS初步问卷。自2017年1月至2019年6月,在定期安排的诊所预约期间,从乔治亚州亚特兰大市的埃默里大学和亚特兰大VA医疗中心的ALS诊所招募了ALS患者,以完成调查表草案和标准的ALS结局指标。募集期间在埃默里大学和亚特兰大弗吉尼亚州医疗中心ALS诊所见过的所有能够诊断出ALS且能够提供知情同意的连续患者均被邀请参加研究。进行了Rasch分析,并根据缺失的数据,模型拟合,阈值无序,项目偏倚和临床判断系统地删除了项目。在募集期间,在这两个地点总共观察到509例ALS患者,并且264例患者提供了知情同意。干预参与者完成了Rasch问卷草稿和修订的肌萎缩性侧索硬化功能评定量表(ALSFRS-R)。主要结果和措施进行Rasch分析和标准量度指标以创建新的量表,并在ALSFRS-R上进行Rasch分析以进行比较。结果总体上,有243名ALS参与者完成了问卷草稿,其中230名参与者参与了Rasch分析。研究参与者的平均(SD)年龄为61.9(11.1)岁,男性为146(60.1%),发病部位为23.0%延髓(n = 56),36.2%上肢(n = 88)和39.5下肢%(n = 96)。构建了28个问题的Rasch-Built总体ALS残疾量表(ROADS),每个项目的评分分别为0、1或2。ROADS符合Rasch模型的要求,与ALSFRS-R相比,具有更强的项目针对性,并且进行了重新测试可靠性为0.97。各个问题的拟合统计显示,拟合值在0.68至1.37之间,服装值在0.66至1.43之间。测度解释的经验方差与模型方差之间的差异为0.1%。ALSFRS-R违反了Rasch模型的预期,并显示了12个问题中的9个的无序阈值;对于任何总体残疾水平,ALSFRS-R上的48个答案中的13个从来都不是最可能的答案。结论与相关性在这项研究中,与ALSFRS-R相比,具有28个问题的自我报告的ROADS具有线性权重,具有更好的项目针对性,并且具有较高的重测信度,并得到了验证。与ALSFRS-R相比,ROADS可以作为一种有价值且易于使用的结局指标,用于ALS试验和临床中,具有更高的响应度。与ALSFRS-R相比,线性加权的自报告ROADS具有更好的项目针对性,并且具有较高的重试可靠性,并得到了验证。与ALSFRS-R相比,ROADS可以作为一种有价值且易于使用的结局指标,用于ALS试验和临床中,具有更高的响应度。与ALSFRS-R相比,线性加权的自报告ROADS具有更好的项目针对性,并且具有较高的重试可靠性,并得到了验证。与ALSFRS-R相比,ROADS可以作为一种有价值且易于使用的结局指标,用于ALS试验和临床中,具有更高的响应度。
更新日期:2020-04-01
中文翻译:

Rasch建立的整体肌萎缩性侧索硬化病残量表(ROADS)的开发和验证。
重要性肌萎缩性侧索硬化症(ALS)临床试验需要一种具有改善反应性的整体残疾水平的新结局指标。目的描述一种新的自我报告的ALS残疾量表的创建和开发,该量表使用数学严格的Rasch方法改进了项目的针对性和心理计量学特性。设计,设置和参与者基于文献回顾,专家组的临床判断和患者意见,创建了一个包含119个问题的ALS初步问卷。自2017年1月至2019年6月,在定期安排的诊所预约期间,从乔治亚州亚特兰大市的埃默里大学和亚特兰大VA医疗中心的ALS诊所招募了ALS患者,以完成调查表草案和标准的ALS结局指标。募集期间在埃默里大学和亚特兰大弗吉尼亚州医疗中心ALS诊所见过的所有能够诊断出ALS且能够提供知情同意的连续患者均被邀请参加研究。进行了Rasch分析,并根据缺失的数据,模型拟合,阈值无序,项目偏倚和临床判断系统地删除了项目。在募集期间,在这两个地点总共观察到509例ALS患者,并且264例患者提供了知情同意。干预参与者完成了Rasch问卷草稿和修订的肌萎缩性侧索硬化功能评定量表(ALSFRS-R)。主要结果和措施进行Rasch分析和标准量度指标以创建新的量表,并在ALSFRS-R上进行Rasch分析以进行比较。结果总体上,有243名ALS参与者完成了问卷草稿,其中230名参与者参与了Rasch分析。研究参与者的平均(SD)年龄为61.9(11.1)岁,男性为146(60.1%),发病部位为23.0%延髓(n = 56),36.2%上肢(n = 88)和39.5下肢%(n = 96)。构建了28个问题的Rasch-Built总体ALS残疾量表(ROADS),每个项目的评分分别为0、1或2。ROADS符合Rasch模型的要求,与ALSFRS-R相比,具有更强的项目针对性,并且进行了重新测试可靠性为0.97。各个问题的拟合统计显示,拟合值在0.68至1.37之间,服装值在0.66至1.43之间。测度解释的经验方差与模型方差之间的差异为0.1%。ALSFRS-R违反了Rasch模型的预期,并显示了12个问题中的9个的无序阈值;对于任何总体残疾水平,ALSFRS-R上的48个答案中的13个从来都不是最可能的答案。结论与相关性在这项研究中,与ALSFRS-R相比,具有28个问题的自我报告的ROADS具有线性权重,具有更好的项目针对性,并且具有较高的重测信度,并得到了验证。与ALSFRS-R相比,ROADS可以作为一种有价值且易于使用的结局指标,用于ALS试验和临床中,具有更高的响应度。与ALSFRS-R相比,线性加权的自报告ROADS具有更好的项目针对性,并且具有较高的重试可靠性,并得到了验证。与ALSFRS-R相比,ROADS可以作为一种有价值且易于使用的结局指标,用于ALS试验和临床中,具有更高的响应度。与ALSFRS-R相比,线性加权的自报告ROADS具有更好的项目针对性,并且具有较高的重试可靠性,并得到了验证。与ALSFRS-R相比,ROADS可以作为一种有价值且易于使用的结局指标,用于ALS试验和临床中,具有更高的响应度。


























 京公网安备 11010802027423号
京公网安备 11010802027423号