Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural Kinetics of MsbA Investigated by Stopped-Flow Time-Resolved Small-Angle X-Ray Scattering.
Structure ( IF 5.7 ) Pub Date : 2019-12-17 , DOI: 10.1016/j.str.2019.12.001 Inokentijs Josts 1 , Yunyun Gao 2 , Diana C F Monteiro 2 , Stephan Niebling 3 , Julius Nitsche 4 , Katharina Veith 1 , Tobias W Gräwert 5 , Clement E Blanchet 5 , Martin A Schroer 5 , Nils Huse 2 , Arwen R Pearson 2 , Dmitri I Svergun 5 , Henning Tidow 1
Structure ( IF 5.7 ) Pub Date : 2019-12-17 , DOI: 10.1016/j.str.2019.12.001 Inokentijs Josts 1 , Yunyun Gao 2 , Diana C F Monteiro 2 , Stephan Niebling 3 , Julius Nitsche 4 , Katharina Veith 1 , Tobias W Gräwert 5 , Clement E Blanchet 5 , Martin A Schroer 5 , Nils Huse 2 , Arwen R Pearson 2 , Dmitri I Svergun 5 , Henning Tidow 1
Affiliation
|
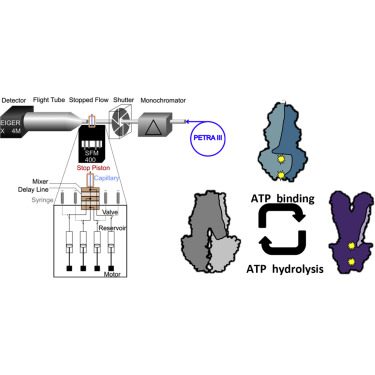
Recent structures of full-length ATP-binding cassette (ABC) transporter MsbA in different states indicate large conformational changes during the reaction cycle that involve transient dimerization of its nucleotide-binding domains (NBDs). However, a detailed molecular understanding of the structural changes and associated kinetics of MsbA upon ATP binding and hydrolysis is still missing. Here, we employed time-resolved small-angle X-ray scattering, initiated by stopped-flow mixing, to investigate the kinetics and accompanying structural changes of NBD dimerization (upon ATP binding) and subsequent dissociation (upon ATP hydrolysis) in the context of isolated NBDs as well as full-length MsbA in lipid nanodiscs. Our data allowed us to structurally characterize the major states involved in the process and determine time constants for NBD dimerization and dissociation. In the full-length protein, these structural transitions occur on much faster time scales, indicating close-proximity effects and structural coupling of the transmembrane domains with the NBDs.
中文翻译:

停止流时间分辨小角X射线散射研究MsbA的结构动力学。
全长ATP结合盒(ABC)转运蛋白MsbA在不同状态下的最新结构表明,在反应周期中,其构象变化涉及其核苷酸结合域(NBD)的瞬时二聚化,其构象变化较大。但是,仍然缺少对MsbA在ATP结合和水解时的结构变化和相关动力学的详细分子理解。在这里,我们采用时间分辨的小角度X射线散射(由停止流混合引发)来研究NBD二聚作用(在ATP结合时)和随后的解离(在ATP水解时)的动力学及其伴随的结构变化。脂质纳米光盘中分离的NBD以及全长MsbA。我们的数据使我们能够从结构上表征过程中涉及的主要状态,并确定NBD二聚和离解的时间常数。在全长蛋白质中,这些结构转变发生的时间要快得多,这表明跨膜结构域与NBD的紧密邻近效应和结构偶联。
更新日期:2019-12-30
中文翻译:

停止流时间分辨小角X射线散射研究MsbA的结构动力学。
全长ATP结合盒(ABC)转运蛋白MsbA在不同状态下的最新结构表明,在反应周期中,其构象变化涉及其核苷酸结合域(NBD)的瞬时二聚化,其构象变化较大。但是,仍然缺少对MsbA在ATP结合和水解时的结构变化和相关动力学的详细分子理解。在这里,我们采用时间分辨的小角度X射线散射(由停止流混合引发)来研究NBD二聚作用(在ATP结合时)和随后的解离(在ATP水解时)的动力学及其伴随的结构变化。脂质纳米光盘中分离的NBD以及全长MsbA。我们的数据使我们能够从结构上表征过程中涉及的主要状态,并确定NBD二聚和离解的时间常数。在全长蛋白质中,这些结构转变发生的时间要快得多,这表明跨膜结构域与NBD的紧密邻近效应和结构偶联。



























 京公网安备 11010802027423号
京公网安备 11010802027423号