当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and properties of a redox-switchable calix[6]arene-based molecular lasso
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2019/12/30 , DOI: 10.1039/c9qo01379b Guido Orlandini 1, 2, 3, 4, 5 , Lorenzo Casimiro 5, 6, 7 , Margherita Bazzoni 1, 2, 3, 4, 5 , Beatrice Cogliati 1, 2, 3, 4, 5 , Alberto Credi 5, 8, 9, 10, 11 , Marco Lucarini 5, 6, 7 , Serena Silvi 5, 6, 7 , Arturo Arduini 1, 2, 3, 4, 5 , Andrea Secchi 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2019/12/30 , DOI: 10.1039/c9qo01379b Guido Orlandini 1, 2, 3, 4, 5 , Lorenzo Casimiro 5, 6, 7 , Margherita Bazzoni 1, 2, 3, 4, 5 , Beatrice Cogliati 1, 2, 3, 4, 5 , Alberto Credi 5, 8, 9, 10, 11 , Marco Lucarini 5, 6, 7 , Serena Silvi 5, 6, 7 , Arturo Arduini 1, 2, 3, 4, 5 , Andrea Secchi 1, 2, 3, 4, 5
Affiliation
|
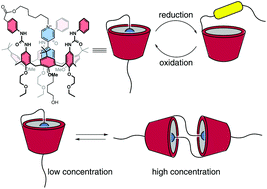
The synthesis and characterisation of calix[6]arene-based lasso-like molecular structures is described. These interwoven structures consist of an electrochemical responsive N,N′-dialkylviologen arm covalently anchored at the upper rim of a triphenylureido calix[6]arene-based wheel. Upon reduction of the viologen core, a hollow tridimensional macrocyclic structure can be generated. This process is reversible, and the original lasso-like structure can be regenerated by oxidizing the viologen arm to its original dicationic form. Electrochemical and EPR techniques investigated the ability of the system to perform threading/dethreading movements upon redox switching. The functionalisation of the arm ω-hydroxy ending with a bulky diphenylacetyl group converts the self-threaded structure in a blocked interwoven molecular compound belonging to the class of [1]rotaxanes. The ability to form dimeric structures in the shape of a [c2]daisy chain was also demonstrated, an unprecedented result for calixarene macrocycles.
中文翻译:

氧化还原可切换杯[6]芳烃基分子套索的合成与性能
合成和表征杯[6]芳烃基套索状分子结构的描述。这些交织结构由电化学响应性N,N'-二烷基紫罗兰臂共价锚固在基于三苯基脲基杯[6]芳烃的车轮的上轮缘上。当紫精芯还原时,可以产生空心的三维大环结构。该过程是可逆的,并且可以通过将紫精臂氧化成其原始的药理形式来再生原始的套索状结构。电化学和EPR技术研究了系统在氧化还原切换后执行穿线/脱线运动的能力。以庞大的二苯基乙酰基为末端的臂ω-羟基的官能化转化了属于[1]轮烷类的嵌段交织分子化合物中的自螺纹结构。形成[ c]形状的二聚体结构的能力2]还显示了菊花链,这是杯芳烃大环化合物的空前结果。
更新日期:2020-02-18
中文翻译:

氧化还原可切换杯[6]芳烃基分子套索的合成与性能
合成和表征杯[6]芳烃基套索状分子结构的描述。这些交织结构由电化学响应性N,N'-二烷基紫罗兰臂共价锚固在基于三苯基脲基杯[6]芳烃的车轮的上轮缘上。当紫精芯还原时,可以产生空心的三维大环结构。该过程是可逆的,并且可以通过将紫精臂氧化成其原始的药理形式来再生原始的套索状结构。电化学和EPR技术研究了系统在氧化还原切换后执行穿线/脱线运动的能力。以庞大的二苯基乙酰基为末端的臂ω-羟基的官能化转化了属于[1]轮烷类的嵌段交织分子化合物中的自螺纹结构。形成[ c]形状的二聚体结构的能力2]还显示了菊花链,这是杯芳烃大环化合物的空前结果。


























 京公网安备 11010802027423号
京公网安备 11010802027423号