当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Coumarin-3-formylpyrazoles as 3-carbon synthons in cyclocondensation for the synthesis of spiro-fused pentacyclic spirooxindoles.
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-01-14 , DOI: 10.1039/c9ob02434d Chuan-Wen Lei 1 , Chuan-Bao Zhang 1 , Zhen-Hua Wang 2 , Ke-Xin Xie 3 , Jian-Qiang Zhao 2 , Ming-Qiang Zhou 4 , Xiao-Mei Zhang 4 , Xiao-Ying Xu 4 , Wei-Cheng Yuan 5
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-01-14 , DOI: 10.1039/c9ob02434d Chuan-Wen Lei 1 , Chuan-Bao Zhang 1 , Zhen-Hua Wang 2 , Ke-Xin Xie 3 , Jian-Qiang Zhao 2 , Ming-Qiang Zhou 4 , Xiao-Mei Zhang 4 , Xiao-Ying Xu 4 , Wei-Cheng Yuan 5
Affiliation
|
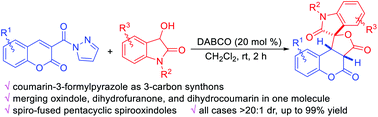
Coumarin-3-formylpyrazoles have been synthesized and applied as 3-carbon synthons in reaction with 3-hydroxyoxindoles by using DABCO as the catalyst. A range of structurally diverse spiro-fused pentacyclic spirooxindoles, bearing a spirooxindole-γ-lactone and a 3,4-dihydrocoumarin substructure, could be smoothly obtained in good to excellent yields (up to 99%) with excellent diastereoselectivities (all cases >20 : 1 dr). The asymmetric version of this tandem reaction was preliminarily investigated by using chiral organocatalysts.
中文翻译:

香豆素-3-甲酰基吡唑类化合物在环缩合中作为3碳合成子用于螺环稠合的五环螺氧并吲哚的合成。
合成了香豆素-3-甲酰基吡唑,并以DABCO为催化剂,将其作为3-碳合成子与3-羟基羟吲哚反应。可以平稳地获得一系列结构多样的螺旋稠合的五环螺旋螺环,它们带有螺氧吲哚-γ-内酯和3,4-二氢香豆素亚结构,具有良好的至优异的产率(高达99%)和非对映选择性(所有情况> 20) :1个博士)。通过使用手性有机催化剂初步研究了该串联反应的不对称形式。
更新日期:2020-02-13
中文翻译:

香豆素-3-甲酰基吡唑类化合物在环缩合中作为3碳合成子用于螺环稠合的五环螺氧并吲哚的合成。
合成了香豆素-3-甲酰基吡唑,并以DABCO为催化剂,将其作为3-碳合成子与3-羟基羟吲哚反应。可以平稳地获得一系列结构多样的螺旋稠合的五环螺旋螺环,它们带有螺氧吲哚-γ-内酯和3,4-二氢香豆素亚结构,具有良好的至优异的产率(高达99%)和非对映选择性(所有情况> 20) :1个博士)。通过使用手性有机催化剂初步研究了该串联反应的不对称形式。


























 京公网安备 11010802027423号
京公网安备 11010802027423号