当前位置:
X-MOL 学术
›
J. Mol. Recognit.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Recognition of the gluconeogenic enzyme, Pck1, via the Gid4 E3 ligase: An in silico perspective.
Journal of Molecular Recognition ( IF 2.7 ) Pub Date : 2019-12-27 , DOI: 10.1002/jmr.2821 Alaa M Ismail 1 , Abdo A Elfiky 1, 2 , Wael M Elshemey 1, 3
Journal of Molecular Recognition ( IF 2.7 ) Pub Date : 2019-12-27 , DOI: 10.1002/jmr.2821 Alaa M Ismail 1 , Abdo A Elfiky 1, 2 , Wael M Elshemey 1, 3
Affiliation
|
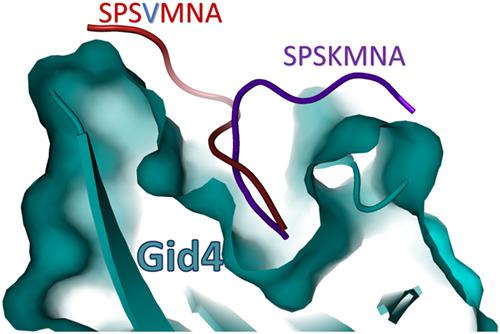
Gluconeogenesis, the reverse process of glycolysis, is a favorable mechanism at conditions of glucose deprivation. Pck1 is a rate-limiting gluconeogenic enzyme, where its deficiency or mutation contributes to serious clinical situations as neonatal hypoglycemia and liver failure. A recent report confirms that Pck1 is a target for proteasomal degradation through its proline residue at the penultimate position, recognized by Gid4 E3 ligase, but with a lack of informative structural details. In this study, we delineate the localized sequence motif, degron, that specifically interact with Gid4 ligase and unravel the binding mode of Pck1 to the Gid4 ligase by using molecular docking and molecular dynamics. The peptide/protein docking HPEPDOCK web server along with molecular dynamic simulations are applied to demonstrate the binding mode and interactions of a Pck1 wild type (SPSK) and mutant (K4V) with the recently solved structure of Gid4 ligase. Results unveil a distinct binding mode of the mutated peptide compared with the wild type despite having comparable binding affinities to Gid4. Moreover, the four-residue peptide is found insufficient for Gid4 binding, while the seven-residue peptide suffices for binding to Gid4. The amino acids S134, K135, and N137 in the loop L1 (between β1 and β2) of the Gid4 are essential for the stabilization of the seven-residue peptide in the binding site of the ligase. The presence of Val4 instead of Lys4 smashes the H-bonds that are formed between Lys4 and Gid4 in the wild type peptide, making the peptide prone to bind with the other side of the binding pocket (L4 loop of Gid4). The dynamics of Gid4 L3 loop is affected dramatically once K4V mutant Pck1 peptide is introduced. This opens the door to explore the mutation effects on the binding mode and smooth the path to target protein degradation by design competitive and non-competitive inhibitors.
中文翻译:

通过Gid4 E3连接酶识别糖原异生酶Pck1:计算机领域的观点。
在糖缺乏的条件下,糖酵解的逆过程即糖原异生是一种有利的机制。Pck1是一种限速的糖异生酶,其中的缺乏或突变会导致严重的临床情况,如新生儿低血糖和肝衰竭。最近的一份报告证实,Pck1通过其倒数第二个位置的脯氨酸残基被蛋白酶裂解,是被Gid4 E3连接酶识别的,但缺乏详细的结构细节。在这项研究中,我们描述了与Gid4连接酶特异性相互作用的局部序列基序degron,并通过分子对接和分子动力学揭示了Pck1与Gid4连接酶的结合模式。肽/蛋白质对接HPEPDOCK Web服务器以及分子动力学模拟被用于证明结合模式以及Pck1野生型(SPSK)和突变体(K4V)与最近解决的Gid4连接酶结构的相互作用。尽管与Gid4具有相似的结合亲和力,但结果揭示了与野生型相比突变肽具有独特的结合模式。此外,发现四个残基的肽不足以与Gid4结合,而七个残基的肽足以与Gid4结合。Gid4的环L1(在β1和β2之间)中的氨基酸S134,K135和N137对于稳定连接酶结合位点中的7个残基肽至关重要。Val4代替Lys4的存在会破坏野生型肽中Lys4和Gid4之间形成的H键,使肽易于与结合袋的另一侧结合(Gid4的L4环)。一旦引入K4V突变体Pck1肽,Gid4 L3环的动力学就会受到显着影响。这为探索突变对结合模式的影响打开了大门,并通过设计竞争性和非竞争性抑制剂为目标蛋白质降解铺平了道路。
更新日期:2020-02-04
中文翻译:

通过Gid4 E3连接酶识别糖原异生酶Pck1:计算机领域的观点。
在糖缺乏的条件下,糖酵解的逆过程即糖原异生是一种有利的机制。Pck1是一种限速的糖异生酶,其中的缺乏或突变会导致严重的临床情况,如新生儿低血糖和肝衰竭。最近的一份报告证实,Pck1通过其倒数第二个位置的脯氨酸残基被蛋白酶裂解,是被Gid4 E3连接酶识别的,但缺乏详细的结构细节。在这项研究中,我们描述了与Gid4连接酶特异性相互作用的局部序列基序degron,并通过分子对接和分子动力学揭示了Pck1与Gid4连接酶的结合模式。肽/蛋白质对接HPEPDOCK Web服务器以及分子动力学模拟被用于证明结合模式以及Pck1野生型(SPSK)和突变体(K4V)与最近解决的Gid4连接酶结构的相互作用。尽管与Gid4具有相似的结合亲和力,但结果揭示了与野生型相比突变肽具有独特的结合模式。此外,发现四个残基的肽不足以与Gid4结合,而七个残基的肽足以与Gid4结合。Gid4的环L1(在β1和β2之间)中的氨基酸S134,K135和N137对于稳定连接酶结合位点中的7个残基肽至关重要。Val4代替Lys4的存在会破坏野生型肽中Lys4和Gid4之间形成的H键,使肽易于与结合袋的另一侧结合(Gid4的L4环)。一旦引入K4V突变体Pck1肽,Gid4 L3环的动力学就会受到显着影响。这为探索突变对结合模式的影响打开了大门,并通过设计竞争性和非竞争性抑制剂为目标蛋白质降解铺平了道路。



























 京公网安备 11010802027423号
京公网安备 11010802027423号