Journal of Energy Chemistry ( IF 13.1 ) Pub Date : 2019-12-28 , DOI: 10.1016/j.jechem.2019.12.023 Han Wu , Min Wu , Boyang Wang , Xue Yong , Yushan Liu , Baojun Li , Baozhong Liu , Siyu Lu
|
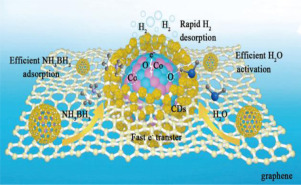
Ammonia borane (AB) is an excellent candidate for the chemical storage of hydrogen. However, its practical utilization for hydrogen production is hindered by the need for expensive noble-metal-based catalysts. Herein, we report Co–Co3O4 nanoparticles (NPs) facilely deposited on carbon dots (CDs) as a highly efficient, robust, and noble-metal-free catalyst for the hydrolysis of AB. The incorporation of the multi-interfaces between Co, Co3O4 NPs, and CDs endows this hybrid material with excellent catalytic activity (rB = 6816 min–1 gCo–1) exceeding that of previous non-noble-metal NP systems and even that of some noble-metal NP systems. A further mechanistic study suggests that these interfacial interactions can affect the electronic structures of interfacial atoms and provide abundant adsorption sites for AB and water molecules, resulting in a low energy barrier for the activation of reactive molecules and thus substantial improvement of the catalytic rate.
中文翻译:

Co-Co 3 O 4 /碳点的界面电子协同迁移:促进硼烷氨的水解脱氢
硼烷氨(AB)是化学储存氢的极佳选择。但是,由于需要昂贵的贵金属基催化剂而阻碍了其在制氢中的实际利用。在本文中,我们报道了Co-Co 3 O 4纳米颗粒(NPs)轻而易举地沉积在碳点(CDs)上,它是AB水解的高效,坚固且不含贵金属的催化剂。Co,Co 3 O 4 NP和CD之间多界面的结合使这种杂化材料具有出色的催化活性(r B = 6816min –1 g Co –1),超过了以前的非贵金属NP系统甚至某些贵金属NP系统的值。进一步的机理研究表明,这些界面相互作用可以影响界面原子的电子结构,并为AB和水分子提供丰富的吸附位点,从而导致低能垒,无法活化反应分子,从而大大提高了催化速率。


























 京公网安备 11010802027423号
京公网安备 11010802027423号