Journal of Catalysis ( IF 7.3 ) Pub Date : 2019-12-27 , DOI: 10.1016/j.jcat.2019.12.015 Tianwei He , Gurpreet Kour , Xin Mao , Aijun Du
|
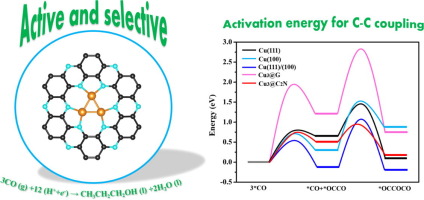
The conversion of carbon monoxide into value-added liquid alcohols has recently attracted considerable attention (Li et al., 2014; De Luna et al., 2018; Pang et al., 2019). However, the ongoing reports for novel catalysts possessing excellent efficiency are still rare due to the high barriers of the reactions involved in the upgradation of the carbon-chains. Cuδ+ sites have been found to promote multi-carbon products, whereas the long-term stability of Cuδ+ sites under reduction conditions remains a challenge for copper-based catalysts. Here, we demonstrate a Mott-Schottky catalyst composed of a copper trimer and the newly synthesized holey C2N semiconductor to stabilize the Cuδ+ sites. The formed metal-semiconductor Schottky barrier promotes electron transfer from the Cu3 cluster to the semiconducting C2N substrate and retains the Cu trimer oxidation state throughout the reaction. The Cu3@C2N catalyst exhibits significantly promoted CC bond formation and hydrogenation activity that outperforms Cu (1 1 1) and Cu (1 0 0) surfaces. In addition, we screened various possible intermediates and provided a plausible thermodynamic reaction pathway for producing n-propanol with a limiting potential of only 0.53 V. Our work offers a promising strategy to maintain the oxidation state of metal sites during the electrocatalysis.
中文翻译:

活性位点稳定化通过莫特-肖特基效应促进一氧化碳的高效转化为Ñ丙醇
一氧化碳向增值液态醇的转化最近引起了相当大的关注(Li等,2014; De Luna等,2018; Pang等,2019)。然而,由于涉及碳链升级的反应的高障碍,关于具有优异效率的新型催化剂的持续报道仍然很少。铜δ+网站被发现,以促进多碳产品,而铜的长期稳定δ+网站还原反应条件下仍是铜基催化剂是一个挑战。在这里,我们演示了由铜三聚体和新合成的有孔C 2 N半导体组成的Mott-Schottky催化剂,可稳定Cuδ+网站。形成的金属半导体肖特基势垒促进电子从Cu 3团簇转移到半导体C 2 N衬底,并在整个反应过程中保持Cu三聚体的氧化态。Cu 3 @C 2 N催化剂表现出显着促进的C C键形成和氢化活性,其性能优于Cu(1 1 1)和Cu(1 0 0)表面。此外,我们筛选了各种可能的中间体,并提供了一个可行的热力学反应途径,用于生产极限电位仅为0.53 V的正丙醇。我们的工作提供了一种在电催化过程中保持金属位点氧化态的有前途的策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号