当前位置:
X-MOL 学术
›
J. Hepatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A randomized, placebo-controlled trial of emricasan in patients with NASH and F1-F3 fibrosis
Journal of Hepatology ( IF 25.7 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.jhep.2019.11.024 Stephen A Harrison 1 , Zachary Goodman 2 , Abdul Jabbar 3 , Ravi Vemulapalli 4 , Ziad H Younes 5 , Bradley Freilich 6 , Muhammad Y Sheikh 7 , Jörn M Schattenberg 8 , Zeid Kayali 9 , Adam Zivony 10 , Aasim Sheikh 11 , Javier Garcia-Samaniego 12 , Sanjaya K Satapathy 13 , George Therapondos 14 , Edward Mena 15 , Detlef Schuppan 16 , James Robinson 17 , Jean L Chan 17 , David T Hagerty 17 , Arun J Sanyal 18
Journal of Hepatology ( IF 25.7 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.jhep.2019.11.024 Stephen A Harrison 1 , Zachary Goodman 2 , Abdul Jabbar 3 , Ravi Vemulapalli 4 , Ziad H Younes 5 , Bradley Freilich 6 , Muhammad Y Sheikh 7 , Jörn M Schattenberg 8 , Zeid Kayali 9 , Adam Zivony 10 , Aasim Sheikh 11 , Javier Garcia-Samaniego 12 , Sanjaya K Satapathy 13 , George Therapondos 14 , Edward Mena 15 , Detlef Schuppan 16 , James Robinson 17 , Jean L Chan 17 , David T Hagerty 17 , Arun J Sanyal 18
Affiliation
|
BACKGROUND AND AIMS
Non-alcoholic steatohepatitis (NASH) is characterized by hepatocyte steatosis, ballooning, and lobular inflammation which may lead to fibrosis. Lipotoxicity activates caspases, which cause apoptosis and inflammatory cytokine (IL-1β and IL-18) production. Emricasan is a pan-caspase inhibitor that decreases serum aminotransferases and caspase activation in NASH patients. This study postulated that 72 weeks of emricasan treatment would improve liver fibrosis without worsening of NASH. METHODS
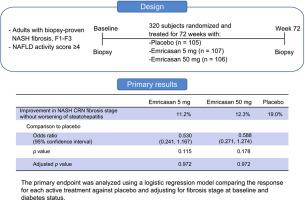
This double-blind, placebo-controlled study randomized 318 subjects 1:1:1 to twice-daily treatment with emricasan (5 or 50 mg) or matching placebo for 72 weeks. Subjects had definite NASH and NASH CRN fibrosis stage F1-F3, as determined by a central reader, on a liver biopsy obtained within 6 months of randomization. RESULTS
Emricasan treatment did not achieve the primary objective of fibrosis improvement without worsening of NASH (emricasan 5 mg: 11.2%; emricasan 50 mg: 12.3%; placebo: 19.0%; odds ratios vs. placebo 0.530 and 0.588, with p=0.972 and 0.972, respectively) or the secondary objective of NASH resolution without worsening of fibrosis (emricasan 5 mg: 3.7%; emricasan 50 mg: 6.6%; placebo: 10.5%; odds ratios vs. placebo 0.334 and 0.613, with p=0.070 and 0.335, respectively). In the small subset of subjects with consistent normalization of serum ALT over 72 weeks, emricasan may have improved histologic outcomes. CONCLUSIONS
Emricasan treatment did not improve liver histology in subjects with NASH fibrosis despite target engagement and may have worsened fibrosis and ballooning. Caspase inhibition lowered serum ALT in the short-term but may have directed cells to alternative mechanisms of cell death, resulting in more liver fibrosis and hepatocyte ballooning. LAY SUMMARY
Non-alcoholic steatohepatitis (NASH) is characterized by fat accumulation in liver cells, which leads to inflammation and fibrosis. Emricasan was previously shown to inhibit some of the liver enzymes which lead to liver inflammation and fibrosis. In this study, emricasan did not improve liver inflammation or fibrosis in patients with NASH and pre-existing liver fibrosis.
中文翻译:

emricasan 在 NASH 和 F1-F3 纤维化患者中的随机、安慰剂对照试验
背景和目的非酒精性脂肪性肝炎(NASH)的特征在于肝细胞脂肪变性、气球样变和小叶炎症,这可能导致纤维化。脂毒性会激活半胱天冬酶,从而导致细胞凋亡和炎性细胞因子(IL-1β 和 IL-18)的产生。Emricasan 是一种泛半胱天冬酶抑制剂,可降低 NASH 患者的血清转氨酶和半胱天冬酶活化。该研究假设 72 周的 emricasan 治疗将改善肝纤维化而不会使 NASH 恶化。方法这项双盲、安慰剂对照研究将 318 名受试者以 1:1:1 的比例随机分配至每天两次使用 emricasan(5 或 50 毫克)或匹配的安慰剂治疗 72 周。在随机化后 6 个月内获得的肝活检中,受试者具有明确的 NASH 和 NASH CRN 纤维化阶段 F1-F3,由中央读取器确定。结果 Emricasan 治疗没有达到纤维化改善的主要目标,而不会使 NASH 恶化(emricasan 5 mg:11.2%;emricasan 50 mg:12.3%;安慰剂:19.0%;与安慰剂的比值比为 0.530 和 0.588,p=0.972分别为 0.972)或 NASH 消退的次要目标,而不会使纤维化恶化(emricasan 5 mg:3.7%;emricasan 50 mg:6.6%;安慰剂:10.5%;与安慰剂的比值比为 0.334 和 0.613,p=0.035 和 0. , 分别)。在 72 周内血清 ALT 持续正常化的一小部分受试者中,emricasan 可能改善了组织学结果。结论 Emricasan 治疗并未改善 NASH 纤维化受试者的肝脏组织学,尽管靶向治疗,并且可能使纤维化和气球样变恶化。Caspase 抑制在短期内降低了血清 ALT,但可能会将细胞引导至细胞死亡的替代机制,导致更多的肝纤维化和肝细胞膨胀。概述 非酒精性脂肪性肝炎 (NASH) 的特征是肝细胞中的脂肪堆积,从而导致炎症和纤维化。Emricasan 以前被证明可以抑制一些导致肝脏炎症和纤维化的肝酶。在这项研究中,emricasan 没有改善 NASH 和预先存在的肝纤维化患者的肝脏炎症或纤维化。Emricasan 以前被证明可以抑制一些导致肝脏炎症和纤维化的肝酶。在这项研究中,emricasan 没有改善 NASH 和预先存在的肝纤维化患者的肝脏炎症或纤维化。Emricasan 以前被证明可以抑制一些导致肝脏炎症和纤维化的肝酶。在这项研究中,emricasan 没有改善 NASH 和预先存在的肝纤维化患者的肝脏炎症或纤维化。
更新日期:2020-05-01
中文翻译:

emricasan 在 NASH 和 F1-F3 纤维化患者中的随机、安慰剂对照试验
背景和目的非酒精性脂肪性肝炎(NASH)的特征在于肝细胞脂肪变性、气球样变和小叶炎症,这可能导致纤维化。脂毒性会激活半胱天冬酶,从而导致细胞凋亡和炎性细胞因子(IL-1β 和 IL-18)的产生。Emricasan 是一种泛半胱天冬酶抑制剂,可降低 NASH 患者的血清转氨酶和半胱天冬酶活化。该研究假设 72 周的 emricasan 治疗将改善肝纤维化而不会使 NASH 恶化。方法这项双盲、安慰剂对照研究将 318 名受试者以 1:1:1 的比例随机分配至每天两次使用 emricasan(5 或 50 毫克)或匹配的安慰剂治疗 72 周。在随机化后 6 个月内获得的肝活检中,受试者具有明确的 NASH 和 NASH CRN 纤维化阶段 F1-F3,由中央读取器确定。结果 Emricasan 治疗没有达到纤维化改善的主要目标,而不会使 NASH 恶化(emricasan 5 mg:11.2%;emricasan 50 mg:12.3%;安慰剂:19.0%;与安慰剂的比值比为 0.530 和 0.588,p=0.972分别为 0.972)或 NASH 消退的次要目标,而不会使纤维化恶化(emricasan 5 mg:3.7%;emricasan 50 mg:6.6%;安慰剂:10.5%;与安慰剂的比值比为 0.334 和 0.613,p=0.035 和 0. , 分别)。在 72 周内血清 ALT 持续正常化的一小部分受试者中,emricasan 可能改善了组织学结果。结论 Emricasan 治疗并未改善 NASH 纤维化受试者的肝脏组织学,尽管靶向治疗,并且可能使纤维化和气球样变恶化。Caspase 抑制在短期内降低了血清 ALT,但可能会将细胞引导至细胞死亡的替代机制,导致更多的肝纤维化和肝细胞膨胀。概述 非酒精性脂肪性肝炎 (NASH) 的特征是肝细胞中的脂肪堆积,从而导致炎症和纤维化。Emricasan 以前被证明可以抑制一些导致肝脏炎症和纤维化的肝酶。在这项研究中,emricasan 没有改善 NASH 和预先存在的肝纤维化患者的肝脏炎症或纤维化。Emricasan 以前被证明可以抑制一些导致肝脏炎症和纤维化的肝酶。在这项研究中,emricasan 没有改善 NASH 和预先存在的肝纤维化患者的肝脏炎症或纤维化。Emricasan 以前被证明可以抑制一些导致肝脏炎症和纤维化的肝酶。在这项研究中,emricasan 没有改善 NASH 和预先存在的肝纤维化患者的肝脏炎症或纤维化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号