当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effect of Mixed-Solvent Environments on the Selectivity of Acid-Catalyzed Dehydration Reactions
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-01-09 , DOI: 10.1021/acscatal.9b03460 Alex K. Chew , Theodore W. Walker , Zhizhang Shen , Benginur Demir , Liam Witteman , Jack Euclide , George W. Huber , James A. Dumesic , Reid C. Van Lehn
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-01-09 , DOI: 10.1021/acscatal.9b03460 Alex K. Chew , Theodore W. Walker , Zhizhang Shen , Benginur Demir , Liam Witteman , Jack Euclide , George W. Huber , James A. Dumesic , Reid C. Van Lehn
|
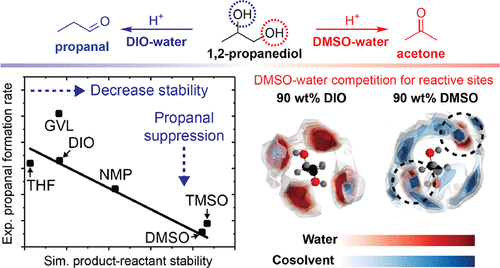
The composition of the liquid phase can alter the rates of individual reaction steps and thus alter the selectivity of acid-catalyzed reactions, but these solvent effects are difficult to anticipate for design purposes. Herein, we report the kinetics and selectivity of Brønsted acid-catalyzed 1,2-propanediol dehydration in pure water and in aqueous mixtures of the polar aprotic cosolvents γ-valerolactone, 1,4-dioxane, tetrahydrofuran, N-methyl-2-pyrrolidone, tetramethylene sulfoxide, and dimethyl sulfoxide at 433 K. We find that the major product of 1,2-propanediol dehydration is propanal in most mixed-solvent environments with selectivities between 1 and 68 mol %. In contrast, 1,2-propanediol dehydration in aqueous mixtures of dimethyl sulfoxide affords acetone as the major product with up to 48% selectivity with minimal propanal formation. We use classical molecular dynamics simulations to probe these solvent effects by computing the difference between the solvation free energies of 1,2-propanediol and propanal in aqueous mixtures of polar aprotic cosolvents and in pure water. We find that the difference in the solvation free energies is correlated with the rates of propanal formation in all mixed-solvent environments, indicating that the solvent-mediated stabilization of the product state relative to the reactant state translates to increased selectivity toward the same product. Similar agreement between simulated solvation free energies and experimental reaction rates/selectivities is established for the acid-catalyzed dehydration of cis- and trans-1,2-cyclohexanediol and 1,3-cyclohexanediol. Finally, analysis of the solvation environment around 1,2-propanediol shows that dimethyl sulfoxide uniquely competes against water to solvate reactive hydroxyl groups, which causes a change in reaction mechanism in this solvent system that leads to the formation of acetone rather than propanal. These results represent a step toward the computationally efficient screening of solvent systems for acid-catalyzed, liquid-phase processes.
中文翻译:

混合溶剂环境对酸催化脱水反应选择性的影响
液相的组成可以改变各个反应步骤的速率,从而改变酸催化反应的选择性,但是对于设计目的,这些溶剂效果很难预料到。在此,我们报道了布朗斯台德酸催化的1,2-丙二醇在纯水和极性非质子助溶剂γ-戊内酯,1,4-二恶烷,四氢呋喃,N的水性混合物中的脱水反应的动力学和选择性。-甲基-2-吡咯烷酮,四亚甲基亚砜和二甲基亚砜在433K。我们发现1,2-丙二醇脱水的主要产物是丙醛,在大多数混合溶剂环境中,选择性为1至68 mol%。相反,在二甲基亚砜的水性混合物中进行的1,2-丙二醇脱水制得的丙酮为主要产物,选择性高达48%,而丙醛的生成最少。我们通过计算极性非质子助溶剂的水性混合物和纯净水中1,2-丙二醇和丙醛的溶剂化自由能之间的差异,使用经典的分子动力学模拟来探索这些溶剂效应。我们发现,在所有混合溶剂环境中,溶剂化自由能的差异与丙醛形成速率相关,这表明产物状态相对于反应物状态的溶剂介导的稳定转化为对相同产物的增加的选择性。模拟的溶剂化自由能和实验反应速率/选择性之间的相似一致性被建立用于酸催化的脱水反应。顺式和反式-1,2-环己二醇和1,3-环己二醇。最后,对1,2-丙二醇周围溶剂化环境的分析表明,二甲基亚砜与水竞争,从而使反应性羟基溶剂化,这导致该溶剂体系中反应机理发生变化,从而导致形成丙酮而不是丙醛。这些结果代表了针对酸催化液相过程的溶剂体系的计算有效筛选的迈出的一步。
更新日期:2020-01-10
中文翻译:

混合溶剂环境对酸催化脱水反应选择性的影响
液相的组成可以改变各个反应步骤的速率,从而改变酸催化反应的选择性,但是对于设计目的,这些溶剂效果很难预料到。在此,我们报道了布朗斯台德酸催化的1,2-丙二醇在纯水和极性非质子助溶剂γ-戊内酯,1,4-二恶烷,四氢呋喃,N的水性混合物中的脱水反应的动力学和选择性。-甲基-2-吡咯烷酮,四亚甲基亚砜和二甲基亚砜在433K。我们发现1,2-丙二醇脱水的主要产物是丙醛,在大多数混合溶剂环境中,选择性为1至68 mol%。相反,在二甲基亚砜的水性混合物中进行的1,2-丙二醇脱水制得的丙酮为主要产物,选择性高达48%,而丙醛的生成最少。我们通过计算极性非质子助溶剂的水性混合物和纯净水中1,2-丙二醇和丙醛的溶剂化自由能之间的差异,使用经典的分子动力学模拟来探索这些溶剂效应。我们发现,在所有混合溶剂环境中,溶剂化自由能的差异与丙醛形成速率相关,这表明产物状态相对于反应物状态的溶剂介导的稳定转化为对相同产物的增加的选择性。模拟的溶剂化自由能和实验反应速率/选择性之间的相似一致性被建立用于酸催化的脱水反应。顺式和反式-1,2-环己二醇和1,3-环己二醇。最后,对1,2-丙二醇周围溶剂化环境的分析表明,二甲基亚砜与水竞争,从而使反应性羟基溶剂化,这导致该溶剂体系中反应机理发生变化,从而导致形成丙酮而不是丙醛。这些结果代表了针对酸催化液相过程的溶剂体系的计算有效筛选的迈出的一步。


























 京公网安备 11010802027423号
京公网安备 11010802027423号