当前位置:
X-MOL 学术
›
J. Inorg. Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of terpolymer-lipid encapsulated diruthenium(II,III)-anti-inflammatory metallodrug nanoparticles to enhance activity against glioblastoma cancer cells.
Journal of Inorganic Biochemistry ( IF 3.9 ) Pub Date : 2019-12-27 , DOI: 10.1016/j.jinorgbio.2019.110984 Samara Rodrigues Alves 1 , Alison Colquhoun 2 , Xiao Yu Wu 3 , Denise de Oliveira Silva 4
Journal of Inorganic Biochemistry ( IF 3.9 ) Pub Date : 2019-12-27 , DOI: 10.1016/j.jinorgbio.2019.110984 Samara Rodrigues Alves 1 , Alison Colquhoun 2 , Xiao Yu Wu 3 , Denise de Oliveira Silva 4
Affiliation
|
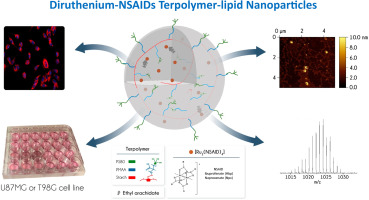
Novel formulations of diruthenium(II,III)-NSAID (NSAID, non-steroidal anti-inflammatory drug) metallodrugs encapsulated into biocompatible terpolymer-lipid nanoparticles (TPLNs) to target glioblastoma cancer were developed. The nanoformulations of Ibuprofenate (RuIbp) and Naproxenate (RuNpx) metallodrugs were synthesized and characterized. The procedure rationally designed to avoid structural changes on the coordination sphere of the [Ru2(NSAID)4]+ paddlewheel unit succeeded in giving colloidally stable and nearly spherical shaped loaded [Ru2(NSAID)4]-TPLNs with appropriate parameters (~90% loading efficiency; drug loading around 10%; particle size ~130 nm; zeta potential around - 40 mV). The maintenance of the [Ru2(NSAID)4]+ framework was confirmed by spectroscopy and mass spectrometry. The encapsulation enhanced antiproliferative effects in U87MG cells for both metallodrugs. The RuIbp-TPLNs showed efficacy also against the cisplatin chemoresistant T98G cancer cells. Lack of significant effects for the loaded-Ibuprofen-TPLNs (HIbp-TPLNs) on both types of cells supports the key role of the dimetal core in the anticancer activity of the [Ru2(NSAID)4]+ metallodrugs. The high cell viability (>70%) found for both types of cells suggests activity associated mainly to antiproliferative effects. The blank-TPLNs internalized into U87MG cell cytoplasm mostly at the first 6 h, by energy-dependent mechanism. The cell uptake of the RuIbp-TPLNs occurred during the first 24 h and it was enhanced in relation to the non-encapsulated metallodrug. The development of these novel metallodrug-loaded TPLN nanoformulations, which exhibit colloidal stability suitable for intravenous injection and enhanced drug cellular uptake, expands the perspective for diruthenium(II,III)-NSAID metallodrugs targeting brain glioblastoma cancer.
中文翻译:

三元共聚物脂质包裹的二钌(II,III)-抗炎金属药物纳米颗粒的合成,以增强针对胶质母细胞瘤癌细胞的活性。
开发了被包裹到生物相容性三元共聚物-脂质纳米颗粒(TPLNs)中的靶向神经胶质母细胞瘤的二钌(II,III)-NSAID(NSAID,非甾体类抗炎药)金属药物的新型制剂。合成并表征了布洛芬酸盐(RuIbp)和萘普生酸盐(RuNpx)金属药物的纳米制剂。合理设计的程序避免了[Ru2(NSAID)4] +桨轮单元协调域上的结构变化,成功地为胶体稳定且接近球形的[Ru2(NSAID)4] -TPLNs提供了适当的参数(〜90%载药效率;药物载量约10%;粒径约130 nm;ζ电位约-40 mV)。[Ru2(NSAID)4] +框架的维护已通过光谱法和质谱法确认。两种金属药物的包封都增强了U87MG细胞的抗增殖作用。RuIbp-TPLNs也显示出对顺铂化学耐药性T98G癌细胞的功效。负载的布洛芬-TPLNs(HIbp-TPLNs)对两种类型的细胞均无明显作用,这支持了双金属核心在[Ru2(NSAID)4] +金属药物的抗癌活性中的关键作用。两种细胞均具有较高的细胞活力(> 70%),表明其活性主要与抗增殖作用有关。通过能量依赖机制,空白TPLNs大部分在最初的6 h内化到U87MG细胞的细胞质中。RuIbp-TPLNs的细胞摄取发生在最初的24小时内,并且相对于未包封的金属药物而言,摄取量有所增加。这些新型的载有金属药物的TPLN纳米制剂的开发,
更新日期:2019-12-27
中文翻译:

三元共聚物脂质包裹的二钌(II,III)-抗炎金属药物纳米颗粒的合成,以增强针对胶质母细胞瘤癌细胞的活性。
开发了被包裹到生物相容性三元共聚物-脂质纳米颗粒(TPLNs)中的靶向神经胶质母细胞瘤的二钌(II,III)-NSAID(NSAID,非甾体类抗炎药)金属药物的新型制剂。合成并表征了布洛芬酸盐(RuIbp)和萘普生酸盐(RuNpx)金属药物的纳米制剂。合理设计的程序避免了[Ru2(NSAID)4] +桨轮单元协调域上的结构变化,成功地为胶体稳定且接近球形的[Ru2(NSAID)4] -TPLNs提供了适当的参数(〜90%载药效率;药物载量约10%;粒径约130 nm;ζ电位约-40 mV)。[Ru2(NSAID)4] +框架的维护已通过光谱法和质谱法确认。两种金属药物的包封都增强了U87MG细胞的抗增殖作用。RuIbp-TPLNs也显示出对顺铂化学耐药性T98G癌细胞的功效。负载的布洛芬-TPLNs(HIbp-TPLNs)对两种类型的细胞均无明显作用,这支持了双金属核心在[Ru2(NSAID)4] +金属药物的抗癌活性中的关键作用。两种细胞均具有较高的细胞活力(> 70%),表明其活性主要与抗增殖作用有关。通过能量依赖机制,空白TPLNs大部分在最初的6 h内化到U87MG细胞的细胞质中。RuIbp-TPLNs的细胞摄取发生在最初的24小时内,并且相对于未包封的金属药物而言,摄取量有所增加。这些新型的载有金属药物的TPLN纳米制剂的开发,



























 京公网安备 11010802027423号
京公网安备 11010802027423号