当前位置:
X-MOL 学术
›
Food Hydrocoll.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enhancing the solubility and foam ability of rice glutelin by heat treatment at pH12: Insight into protein structure
Food Hydrocolloids ( IF 10.7 ) Pub Date : 2020-06-01 , DOI: 10.1016/j.foodhyd.2019.105626 Meng Zhao , Wenfei Xiong , Boxuan Chen , Jie Zhu , Lifeng Wang
Food Hydrocolloids ( IF 10.7 ) Pub Date : 2020-06-01 , DOI: 10.1016/j.foodhyd.2019.105626 Meng Zhao , Wenfei Xiong , Boxuan Chen , Jie Zhu , Lifeng Wang
|
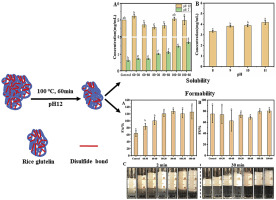
Abstract Glutelin in the rice protein content of up to 80% (dry basis), due to its alkali-soluble protein, severely limited the application of rice protein, a high-quality hypoallergenic plant protein, as an ingredient in the food industry. Herein, rice glutelin dissolved in alkaline solution (pH12) was heat treated for 30 and 60 min at different temperatures (60, 80, 100 °C). It was found that the solubility of glutelin at pH7 after heating at 100 °C for 60 min increased by about 3 times compared with native protein, as well as foaming ability increased by about 2 times and exhibited good foam stability. Dynamic light scattering (DLS) and atomic force microscopy (AFM) results showed that the heat treatment (100 °C, 60 min) resulted in a decrease in the size (about 100 nm) of globular glutelin aggregates, accompanied by a reduction in disulfide bond content and an increase in surface net potential and hydrophobicity. Sodium dodecyl sulfate–polyacrylamide gel (SDS–PAGE) electrophoresis, UV–visible, intrinsic fluorescence and infrared spectroscopy characterization revealed that the primary, tertiary and secondary conformations of glutelin were altered due to heat treatment. In addition, small angle X-ray scattering (SAXS) results suggested that the glutelin aggregates were more flexible after heat treatment. These dimensional and structural changes contributed to the improvement of rice glutelin functional properties after heat treatment, implying that this combination of alkali and heat treatment has potential for enhancing the performance of rice proteins.
中文翻译:

通过 pH12 热处理提高米谷蛋白的溶解性和起泡能力:蛋白质结构的洞察
摘要 大米蛋白中谷蛋白含量高达80%(干基),由于其蛋白质为碱溶性,严重限制了大米蛋白作为一种优质低过敏性植物蛋白在食品工业中的应用。在此,将溶解在碱性溶液 (pH12) 中的米谷蛋白在不同温度 (60、80、100 °C) 下热处理 30 和 60 分钟。发现在100℃加热60分钟后,谷蛋白在pH7下的溶解度比天然蛋白质增加约3倍,发泡能力增加约2倍,表现出良好的泡沫稳定性。动态光散射 (DLS) 和原子力显微镜 (AFM) 结果表明,热处理(100 °C,60 分钟)导致球状谷蛋白聚集体的尺寸减小(约 100 nm),伴随着二硫键含量的减少和表面净电位和疏水性的增加。十二烷基硫酸钠-聚丙烯酰胺凝胶(SDS-PAGE)电泳、紫外-可见光、固有荧光和红外光谱表征表明,谷蛋白的一级、三级和二级构象因热处理而改变。此外,小角 X 射线散射 (SAXS) 结果表明,谷蛋白聚集体在热处理后更加柔韧。这些尺寸和结构的变化有助于热处理后大米谷蛋白功能特性的改善,这意味着碱和热处理的这种结合具有提高大米蛋白质性能的潜力。十二烷基硫酸钠-聚丙烯酰胺凝胶(SDS-PAGE)电泳、紫外-可见光、固有荧光和红外光谱表征表明,谷蛋白的一级、三级和二级构象因热处理而改变。此外,小角 X 射线散射 (SAXS) 结果表明,谷蛋白聚集体在热处理后更加柔韧。这些尺寸和结构的变化有助于热处理后大米谷蛋白功能特性的改善,这意味着碱和热处理的这种结合具有提高大米蛋白质性能的潜力。十二烷基硫酸钠-聚丙烯酰胺凝胶(SDS-PAGE)电泳、紫外-可见光、固有荧光和红外光谱表征表明,谷蛋白的一级、三级和二级构象因热处理而改变。此外,小角 X 射线散射 (SAXS) 结果表明,谷蛋白聚集体在热处理后更加柔韧。这些尺寸和结构的变化有助于热处理后大米谷蛋白功能特性的改善,这意味着碱和热处理的这种结合具有提高大米蛋白质性能的潜力。由于热处理,谷蛋白的三级和二级构象发生了改变。此外,小角 X 射线散射 (SAXS) 结果表明,谷蛋白聚集体在热处理后更加柔韧。这些尺寸和结构的变化有助于热处理后大米谷蛋白功能特性的改善,这意味着碱和热处理的这种结合具有提高大米蛋白质性能的潜力。由于热处理,谷蛋白的三级和二级构象发生了改变。此外,小角 X 射线散射 (SAXS) 结果表明,谷蛋白聚集体在热处理后更加柔韧。这些尺寸和结构的变化有助于热处理后大米谷蛋白功能特性的改善,这意味着碱和热处理的这种结合具有提高大米蛋白质性能的潜力。
更新日期:2020-06-01
中文翻译:

通过 pH12 热处理提高米谷蛋白的溶解性和起泡能力:蛋白质结构的洞察
摘要 大米蛋白中谷蛋白含量高达80%(干基),由于其蛋白质为碱溶性,严重限制了大米蛋白作为一种优质低过敏性植物蛋白在食品工业中的应用。在此,将溶解在碱性溶液 (pH12) 中的米谷蛋白在不同温度 (60、80、100 °C) 下热处理 30 和 60 分钟。发现在100℃加热60分钟后,谷蛋白在pH7下的溶解度比天然蛋白质增加约3倍,发泡能力增加约2倍,表现出良好的泡沫稳定性。动态光散射 (DLS) 和原子力显微镜 (AFM) 结果表明,热处理(100 °C,60 分钟)导致球状谷蛋白聚集体的尺寸减小(约 100 nm),伴随着二硫键含量的减少和表面净电位和疏水性的增加。十二烷基硫酸钠-聚丙烯酰胺凝胶(SDS-PAGE)电泳、紫外-可见光、固有荧光和红外光谱表征表明,谷蛋白的一级、三级和二级构象因热处理而改变。此外,小角 X 射线散射 (SAXS) 结果表明,谷蛋白聚集体在热处理后更加柔韧。这些尺寸和结构的变化有助于热处理后大米谷蛋白功能特性的改善,这意味着碱和热处理的这种结合具有提高大米蛋白质性能的潜力。十二烷基硫酸钠-聚丙烯酰胺凝胶(SDS-PAGE)电泳、紫外-可见光、固有荧光和红外光谱表征表明,谷蛋白的一级、三级和二级构象因热处理而改变。此外,小角 X 射线散射 (SAXS) 结果表明,谷蛋白聚集体在热处理后更加柔韧。这些尺寸和结构的变化有助于热处理后大米谷蛋白功能特性的改善,这意味着碱和热处理的这种结合具有提高大米蛋白质性能的潜力。十二烷基硫酸钠-聚丙烯酰胺凝胶(SDS-PAGE)电泳、紫外-可见光、固有荧光和红外光谱表征表明,谷蛋白的一级、三级和二级构象因热处理而改变。此外,小角 X 射线散射 (SAXS) 结果表明,谷蛋白聚集体在热处理后更加柔韧。这些尺寸和结构的变化有助于热处理后大米谷蛋白功能特性的改善,这意味着碱和热处理的这种结合具有提高大米蛋白质性能的潜力。由于热处理,谷蛋白的三级和二级构象发生了改变。此外,小角 X 射线散射 (SAXS) 结果表明,谷蛋白聚集体在热处理后更加柔韧。这些尺寸和结构的变化有助于热处理后大米谷蛋白功能特性的改善,这意味着碱和热处理的这种结合具有提高大米蛋白质性能的潜力。由于热处理,谷蛋白的三级和二级构象发生了改变。此外,小角 X 射线散射 (SAXS) 结果表明,谷蛋白聚集体在热处理后更加柔韧。这些尺寸和结构的变化有助于热处理后大米谷蛋白功能特性的改善,这意味着碱和热处理的这种结合具有提高大米蛋白质性能的潜力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号