当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Experimental and Thermodynamic Modeling Study of the Quaternary System Containing Lithium, Potassium, Magnesium, and Sulfate at 288.15 K
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2019-12-27 , DOI: 10.1021/acs.jced.9b00731 Shiqiang Wang 1, 2 , Chuncheng Shi 1 , Fei Yuan 1 , Yafei Guo 1, 2 , Tianlong Deng 1, 2
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2019-12-27 , DOI: 10.1021/acs.jced.9b00731 Shiqiang Wang 1, 2 , Chuncheng Shi 1 , Fei Yuan 1 , Yafei Guo 1, 2 , Tianlong Deng 1, 2
Affiliation
|
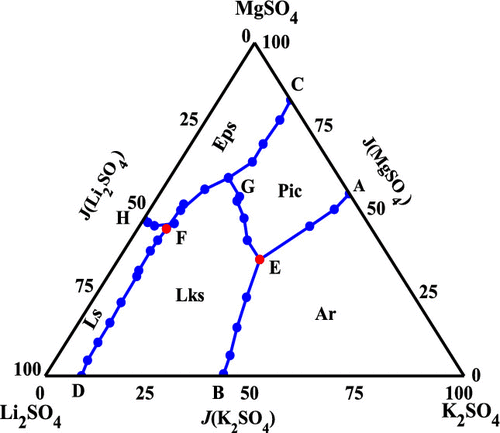
Solid–liquid phase equilibria of the quaternary system (Li + K + Mg + SO4 + H2O) at 288.15 K were investigated with the isothermal dissolution method. The diagrams of solubilities, densities and refractive indices were plotted, respectively. The solubilities diagram of the quaternary system includes three invariant points cosaturated with three salts and five crystallization zones. Two kinds of double salts K2SO4·MgSO4·6H2O and Li2SO4·K2SO4 were found, and no solid solution was formed. Pitzer theory for aqueous electrolyte solution and its extended Harvie-Møller-Weare (HMW) model was used to calculate the solubilities of the quaternary system. A comparison between the calculated and the experimental results shows that the predicted solubilities obtained with the extended HMW model agree well with experimental data.
中文翻译:

288.15 K时含锂,钾,镁和硫酸盐的四元体系的实验和热力学建模研究
用等温溶解法研究了四元体系(Li + K + Mg + SO 4 + H 2 O)在288.15 K时的固液相平衡。分别绘制了溶解度,密度和折射率的图。四元系统的溶解度图包括三个不变点,这些不变点与三个盐和五个结晶区共饱和。两种复盐K 2 SO 4 ·MgSO 4 ·6H 2 O和Li 2 SO 4 ·K 2 SO 4被发现,没有形成固溶体。电解质水溶液的Pitzer理论及其扩展的Harvie-Møller-Weare(HMW)模型用于计算四元体系的溶解度。计算结果与实验结果之间的比较表明,扩展的HMW模型获得的预测溶解度与实验数据吻合良好。
更新日期:2019-12-27
中文翻译:

288.15 K时含锂,钾,镁和硫酸盐的四元体系的实验和热力学建模研究
用等温溶解法研究了四元体系(Li + K + Mg + SO 4 + H 2 O)在288.15 K时的固液相平衡。分别绘制了溶解度,密度和折射率的图。四元系统的溶解度图包括三个不变点,这些不变点与三个盐和五个结晶区共饱和。两种复盐K 2 SO 4 ·MgSO 4 ·6H 2 O和Li 2 SO 4 ·K 2 SO 4被发现,没有形成固溶体。电解质水溶液的Pitzer理论及其扩展的Harvie-Møller-Weare(HMW)模型用于计算四元体系的溶解度。计算结果与实验结果之间的比较表明,扩展的HMW模型获得的预测溶解度与实验数据吻合良好。


























 京公网安备 11010802027423号
京公网安备 11010802027423号