当前位置:
X-MOL 学术
›
BBA Gen. Subj.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The transition between active and inactive conformations of Abl kinase studied by rock climbing and Milestoning.
Biochimica et Biophysica Acta (BBA) - General Subjects ( IF 3 ) Pub Date : 2019-12-27 , DOI: 10.1016/j.bbagen.2019.129508 Brajesh Narayan 1 , Arman Fathizadeh 2 , Clark Templeton 3 , Peng He 4 , Shima Arasteh 4 , Ron Elber 5 , Nicolae-Viorel Buchete 1 , Ron M Levy 4
Biochimica et Biophysica Acta (BBA) - General Subjects ( IF 3 ) Pub Date : 2019-12-27 , DOI: 10.1016/j.bbagen.2019.129508 Brajesh Narayan 1 , Arman Fathizadeh 2 , Clark Templeton 3 , Peng He 4 , Shima Arasteh 4 , Ron Elber 5 , Nicolae-Viorel Buchete 1 , Ron M Levy 4
Affiliation
|
BACKGROUND
Kinases are a family of enzymes that catalyze the transfer of the ɤ-phosphate group from ATP to a protein's residue. Malfunctioning kinases are involved in many health problems such as cardiovascular diseases, diabetes, and cancer. Kinases transitions between multiple conformations of inactive to active forms attracted considerable interest.
METHOD
A reaction coordinate is computed for the transition between the active to inactive conformation in Abl kinase with a focus on the DFG-in to DFG-out flip. The method of Rock Climbing is used to construct a path locally, which is subsequently optimized using a functional of the entire path. The discrete coordinate sets along the reaction path are used in a Milestoning calculation of the free energy landscape and the rate of the transition.
RESULTS
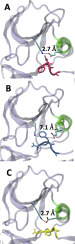
The estimated transition times are between a few milliseconds and seconds, consistent with simulations of the kinetics and with indirect experimental data. The activation requires the transient dissociation of the salt bridge between Lys271 and Glu286. The salt bridge reforms once the DFG motif is stabilized by a locked conformation of Phe382. About ten residues are identified that contribute significantly to the process and are included as part of the reaction space.
CONCLUSIONS
The transition from DFG-in to DFG-out in Abl kinase was simulated using atomic resolution of a fully solvated protein yielding detailed description of the kinetics and the mechanism of the DFG flip. The results are consistent with other computational methods that simulate the kinetics and with some indirect experimental measurements.
GENERAL SIGNIFICANCE
The activation of kinases includes a conformational transition of the DFG motif that is important for enzyme activity but is not accessible to conventional Molecular Dynamics. We propose a detailed mechanism for the transition, at a timescale longer than conventional MD, using a combination of reaction path and Milestoning algorithms. The mechanism includes local structural adjustments near the binding site as well as collective interactions with more remote residues.
中文翻译:

通过攀岩和里程碑研究 Abl 激酶活性和非活性构象之间的转变。
背景激酶是催化ɤ-磷酸基团从ATP转移到蛋白质残基的酶家族。功能失调的激酶与许多健康问题有关,例如心血管疾病、糖尿病和癌症。激酶从非活性形式到活性形式的多种构象之间的转变引起了人们极大的兴趣。方法 计算 Abl 激酶中活性构象到非活性构象之间转变的反应坐标,重点关注 DFG-in 到 DFG-out 的翻转。攀岩方法用于在本地构建路径,随后使用整个路径的函数对其进行优化。沿着反应路径的离散坐标集用于自由能景观和转变速率的里程碑计算。结果估计的转变时间在几毫秒到几秒之间,与动力学模拟和间接实验数据一致。激活需要 Lys271 和 Glu286 之间的盐桥瞬时解离。一旦 DFG 基序被 Phe382 的锁定构象稳定下来,盐桥就会重新形成。鉴定出大约十种残留物,这些残留物对该过程有显着贡献,并作为反应空间的一部分包含在内。结论 使用完全溶剂化蛋白质的原子分辨率模拟 Abl 激酶中从 DFG-in 到 DFG-out 的转变,从而详细描述 DFG 翻转的动力学和机制。结果与模拟动力学的其他计算方法以及一些间接实验测量结果一致。一般意义 激酶的激活包括 DFG 基序的构象转变,这对于酶活性很重要,但传统的分子动力学无法实现。我们结合反应路径和里程碑算法,提出了一种详细的转变机制,其时间尺度比传统 MD 更长。该机制包括结合位点附近的局部结构调整以及与更远的残基的集体相互作用。
更新日期:2019-12-27
中文翻译:

通过攀岩和里程碑研究 Abl 激酶活性和非活性构象之间的转变。
背景激酶是催化ɤ-磷酸基团从ATP转移到蛋白质残基的酶家族。功能失调的激酶与许多健康问题有关,例如心血管疾病、糖尿病和癌症。激酶从非活性形式到活性形式的多种构象之间的转变引起了人们极大的兴趣。方法 计算 Abl 激酶中活性构象到非活性构象之间转变的反应坐标,重点关注 DFG-in 到 DFG-out 的翻转。攀岩方法用于在本地构建路径,随后使用整个路径的函数对其进行优化。沿着反应路径的离散坐标集用于自由能景观和转变速率的里程碑计算。结果估计的转变时间在几毫秒到几秒之间,与动力学模拟和间接实验数据一致。激活需要 Lys271 和 Glu286 之间的盐桥瞬时解离。一旦 DFG 基序被 Phe382 的锁定构象稳定下来,盐桥就会重新形成。鉴定出大约十种残留物,这些残留物对该过程有显着贡献,并作为反应空间的一部分包含在内。结论 使用完全溶剂化蛋白质的原子分辨率模拟 Abl 激酶中从 DFG-in 到 DFG-out 的转变,从而详细描述 DFG 翻转的动力学和机制。结果与模拟动力学的其他计算方法以及一些间接实验测量结果一致。一般意义 激酶的激活包括 DFG 基序的构象转变,这对于酶活性很重要,但传统的分子动力学无法实现。我们结合反应路径和里程碑算法,提出了一种详细的转变机制,其时间尺度比传统 MD 更长。该机制包括结合位点附近的局部结构调整以及与更远的残基的集体相互作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号