Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Preparation of Silica@Silica Core-Shell Microspheres Using an Aqueous Two-Phase System in a Novel Microchannel Device.
Langmuir ( IF 3.9 ) Pub Date : 2020-01-09 , DOI: 10.1021/acs.langmuir.9b03034 Jie Li 1 , Feng Zhang 1 , Leilei Jiang 1 , Liang Yu 2 , Lixiong Zhang 1
Langmuir ( IF 3.9 ) Pub Date : 2020-01-09 , DOI: 10.1021/acs.langmuir.9b03034 Jie Li 1 , Feng Zhang 1 , Leilei Jiang 1 , Liang Yu 2 , Lixiong Zhang 1
Affiliation
|
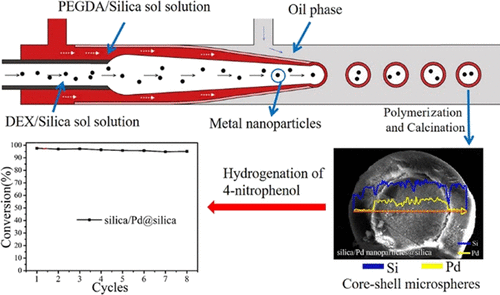
In the present work, a novel microchannel device was developed and used for the preparation of core-shell microspheres combining with a dextran/poly(ethylene glycol) diacrylate (DEX/PEGDA) aqueous two-phase system. Silica@silica core-shell microspheres were prepared as a model material. Silica@silica core-shell microspheres with different sizes of cores and thicknesses of shells were prepared by using different flowrate ratios of DEX/silica and PEGDA/silica aqueous solutions. The content of colloidal silica and the calcination temperature have a significant effect on the texture properties of the prepared core-shell microspheres. The surface area decreased from 199 to 177 m2/g with an increase in the colloidal silica content from 30 to 60 wt %. For a specific colloidal silica content (50 wt %), with the increase in calcination temperature from room temperature to 650 °C, the total pore volume went through a maximum of 0.7 cm3 g-1 with a surface area of 178 m2 g-1 and pore size of 7.32 nm at 450 °C. Due to the accumulation of metal nanoparticles in DEX, different metal nanoparticles (Ni and Pd) were successfully introduced into the core of the core-shell microspheres for the preparation of silica/metal nanoparticles@silica core-shell microsphere catalysts. The catalysts showed similar catalytic performance as the metal nanoparticles for hydrogenation of 4-nitrophenol with a conversion higher than 95%. However, the core-shell microsphere catalyst is much easier to recover. The reuse experiments indicated that the core-shell catalyst has high stability.
中文翻译:

在新型微通道装置中使用水相两相系统制备Silica @ Silica核壳微球。
在目前的工作中,开发了一种新颖的微通道装置,并与葡聚糖/聚(乙二醇)二丙烯酸酯(DEX / PEGDA)水相两相系统结合用于制备核壳微球。制备了二氧化硅@二氧化硅核-壳微球作为模型材料。通过使用不同的DEX /二氧化硅和PEGDA /二氧化硅水溶液的流速比,制备了具有不同核尺寸和壳厚度的二氧化硅@二氧化硅核-壳微球。胶态二氧化硅的含量和煅烧温度对所制备的核-壳微球的织构性质具有显着影响。随着胶态二氧化硅含量从30wt%增加到60wt%,表面积从199减少到177m2 / g。对于特定的胶体二氧化硅含量(50 wt%),随着煅烧温度从室温升高到650°C,在450°C时,总孔体积最大通过0.7 cm3 g-1,表面积为178 m2 g-1,孔径为7.32 nm。由于金属纳米粒子在DEX中的积累,已成功地将不同的金属纳米粒子(Ni和Pd)引入到核壳微球的核中,以制备二氧化硅/金属纳米粒子@二氧化硅核壳微球催化剂。所述催化剂显示出与金属纳米颗粒相似的催化4-硝基苯酚氢化的催化性能,转化率高于95%。然而,核-壳微球催化剂更容易回收。再利用实验表明,核-壳催化剂具有很高的稳定性。7 cm3 g-1,表面积为178 m2 g-1,在450°C时的孔径为7.32 nm。由于金属纳米粒子在DEX中的积累,已成功地将不同的金属纳米粒子(Ni和Pd)引入到核壳微球的核中,以制备二氧化硅/金属纳米粒子@二氧化硅核壳微球催化剂。所述催化剂显示出与金属纳米颗粒相似的催化4-硝基苯酚氢化的催化性能,转化率高于95%。然而,核-壳微球催化剂更容易回收。再利用实验表明,核-壳催化剂具有很高的稳定性。7 cm3 g-1,表面积为178 m2 g-1,在450°C时的孔径为7.32 nm。由于金属纳米粒子在DEX中的积累,已成功地将不同的金属纳米粒子(Ni和Pd)引入到核壳微球的核中,以制备二氧化硅/金属纳米粒子@二氧化硅核壳微球催化剂。该催化剂显示出与金属纳米粒子相似的催化4-硝基苯酚氢化的催化性能,转化率高于95%。然而,核-壳微球催化剂更容易回收。再利用实验表明,核-壳催化剂具有很高的稳定性。将不同的金属纳米粒子(Ni和Pd)成功引入到核壳微球的核中,以制备二氧化硅/金属纳米粒子@二氧化硅核壳微球催化剂。所述催化剂显示出与金属纳米颗粒相似的催化4-硝基苯酚氢化的催化性能,转化率高于95%。然而,核-壳微球催化剂更容易回收。再利用实验表明,核-壳催化剂具有很高的稳定性。将不同的金属纳米粒子(Ni和Pd)成功引入到核壳微球的核中,以制备二氧化硅/金属纳米粒子@二氧化硅核壳微球催化剂。该催化剂显示出与金属纳米粒子相似的催化4-硝基苯酚氢化的催化性能,转化率高于95%。然而,核-壳微球催化剂更容易回收。再利用实验表明,核-壳催化剂具有很高的稳定性。
更新日期:2020-01-09
中文翻译:

在新型微通道装置中使用水相两相系统制备Silica @ Silica核壳微球。
在目前的工作中,开发了一种新颖的微通道装置,并与葡聚糖/聚(乙二醇)二丙烯酸酯(DEX / PEGDA)水相两相系统结合用于制备核壳微球。制备了二氧化硅@二氧化硅核-壳微球作为模型材料。通过使用不同的DEX /二氧化硅和PEGDA /二氧化硅水溶液的流速比,制备了具有不同核尺寸和壳厚度的二氧化硅@二氧化硅核-壳微球。胶态二氧化硅的含量和煅烧温度对所制备的核-壳微球的织构性质具有显着影响。随着胶态二氧化硅含量从30wt%增加到60wt%,表面积从199减少到177m2 / g。对于特定的胶体二氧化硅含量(50 wt%),随着煅烧温度从室温升高到650°C,在450°C时,总孔体积最大通过0.7 cm3 g-1,表面积为178 m2 g-1,孔径为7.32 nm。由于金属纳米粒子在DEX中的积累,已成功地将不同的金属纳米粒子(Ni和Pd)引入到核壳微球的核中,以制备二氧化硅/金属纳米粒子@二氧化硅核壳微球催化剂。所述催化剂显示出与金属纳米颗粒相似的催化4-硝基苯酚氢化的催化性能,转化率高于95%。然而,核-壳微球催化剂更容易回收。再利用实验表明,核-壳催化剂具有很高的稳定性。7 cm3 g-1,表面积为178 m2 g-1,在450°C时的孔径为7.32 nm。由于金属纳米粒子在DEX中的积累,已成功地将不同的金属纳米粒子(Ni和Pd)引入到核壳微球的核中,以制备二氧化硅/金属纳米粒子@二氧化硅核壳微球催化剂。所述催化剂显示出与金属纳米颗粒相似的催化4-硝基苯酚氢化的催化性能,转化率高于95%。然而,核-壳微球催化剂更容易回收。再利用实验表明,核-壳催化剂具有很高的稳定性。7 cm3 g-1,表面积为178 m2 g-1,在450°C时的孔径为7.32 nm。由于金属纳米粒子在DEX中的积累,已成功地将不同的金属纳米粒子(Ni和Pd)引入到核壳微球的核中,以制备二氧化硅/金属纳米粒子@二氧化硅核壳微球催化剂。该催化剂显示出与金属纳米粒子相似的催化4-硝基苯酚氢化的催化性能,转化率高于95%。然而,核-壳微球催化剂更容易回收。再利用实验表明,核-壳催化剂具有很高的稳定性。将不同的金属纳米粒子(Ni和Pd)成功引入到核壳微球的核中,以制备二氧化硅/金属纳米粒子@二氧化硅核壳微球催化剂。所述催化剂显示出与金属纳米颗粒相似的催化4-硝基苯酚氢化的催化性能,转化率高于95%。然而,核-壳微球催化剂更容易回收。再利用实验表明,核-壳催化剂具有很高的稳定性。将不同的金属纳米粒子(Ni和Pd)成功引入到核壳微球的核中,以制备二氧化硅/金属纳米粒子@二氧化硅核壳微球催化剂。该催化剂显示出与金属纳米粒子相似的催化4-硝基苯酚氢化的催化性能,转化率高于95%。然而,核-壳微球催化剂更容易回收。再利用实验表明,核-壳催化剂具有很高的稳定性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号