当前位置:
X-MOL 学术
›
Sustain. Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Vapor-fed photoelectrolysis of water at 0.3 V using gas-diffusion photoanodes of SrTiO3 layers
Sustainable Energy & Fuels ( IF 5.6 ) Pub Date : 2019/12/26 , DOI: 10.1039/c9se01068h Fumiaki Amano 1, 2, 3, 4, 5 , Hyosuke Mukohara 1, 2, 3, 4 , Hiroki Sato 1, 2, 3, 4 , Chihiro Tateishi 1, 2, 3, 4 , Hiromasa Sato 4, 6, 7, 8 , Toshiki Sugimoto 4, 5, 6, 9, 10
Sustainable Energy & Fuels ( IF 5.6 ) Pub Date : 2019/12/26 , DOI: 10.1039/c9se01068h Fumiaki Amano 1, 2, 3, 4, 5 , Hyosuke Mukohara 1, 2, 3, 4 , Hiroki Sato 1, 2, 3, 4 , Chihiro Tateishi 1, 2, 3, 4 , Hiromasa Sato 4, 6, 7, 8 , Toshiki Sugimoto 4, 5, 6, 9, 10
Affiliation
|
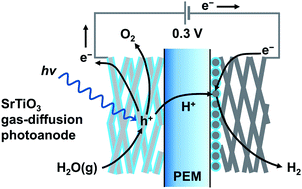
Large scale production of H2 using renewable energy can be realized by photoelectrochemical (PEC) splitting of water vapor in the gas phase. Air humidity can be a water source for producing solar H2. The concept is based on photoelectrochemistry in a gaseous environment using a proton exchange membrane (PEM) as a solid polymer electrolyte and a macroporous semiconductor electrode wrapped in a perfluorosulfonic acid ionomer. A prototype vapor-fed PEC cell using a TiO2 gas-diffusion electrode as an O2-evolving photoanode and a carbon-supported platinum catalyst cathode achieved water splitting to produce O2 and H2, which are physically separated from each other by the PEM. Herein, we demonstrate a PEM-PEC system consisting of a strontium titanate (SrTiO3) nanocrystalline layer decorated on titanium microfiber felt to achieve overall water splitting into H2 and O2 at a ratio of 2 : 1 on each electrode at an applied voltage of only 0.3 V under gaseous conditions. In comparison with TiO2 electrodes, the SrTiO3 gas-diffusion electrode decreased the external voltage and suppressed the gradual decomposition of the ionomer coated on the photoanode. These differences are because of the cathodic shift of the photocurrent onset potential and improvement of the Faraday efficiency of O2 evolution, respectively. The interface between the SrTiO3 surface and the ionomer thin film exhibited fast kinetics for O2 evolution from water adsorbates supplied from the gas phase and durability under ultraviolet light irradiation.
中文翻译:

使用SrTiO3层的气体扩散光阳极在0.3 V下进行水蒸气进给的光电解
可以通过光化学(PEC)分解气相中的水蒸气来实现使用可再生能源大规模生产H 2。空气湿度可以是产生太阳能H 2的水源。该概念基于气态环境中的光电化学,使用质子交换膜(PEM)作为固体聚合物电解质和包裹在全氟磺酸离聚物中的大孔半导体电极。使用TiO 2气体扩散电极作为O 2析出的光电阳极和碳载铂催化剂阴极的原型汽养PEC电池实现了水分解以产生O 2和H 2,它们之间通过PEM物理隔离。在本文中,我们演示了一种PEM-PEC系统,该系统由装饰在钛超细纤维毡上的钛酸锶(SrTiO 3)纳米晶层组成,可在施加电压的情况下在每个电极上以2:1的比例实现总水分解为H 2和O 2在气态条件下仅为0.3V。与TiO 2电极相比,SrTiO 3气体扩散电极降低了外部电压,并抑制了涂覆在光阳极上的离聚物的逐渐分解。这些差异是由于光电流起始电位的阴极移动和O 2的法拉第效率的提高引起的。进化,分别。SrTiO 3表面和离聚物薄膜之间的界面显示出从气相提供的水吸附物中释放出O 2的快速动力学,并在紫外线照射下具有耐久性。
更新日期:2020-03-03
中文翻译:

使用SrTiO3层的气体扩散光阳极在0.3 V下进行水蒸气进给的光电解
可以通过光化学(PEC)分解气相中的水蒸气来实现使用可再生能源大规模生产H 2。空气湿度可以是产生太阳能H 2的水源。该概念基于气态环境中的光电化学,使用质子交换膜(PEM)作为固体聚合物电解质和包裹在全氟磺酸离聚物中的大孔半导体电极。使用TiO 2气体扩散电极作为O 2析出的光电阳极和碳载铂催化剂阴极的原型汽养PEC电池实现了水分解以产生O 2和H 2,它们之间通过PEM物理隔离。在本文中,我们演示了一种PEM-PEC系统,该系统由装饰在钛超细纤维毡上的钛酸锶(SrTiO 3)纳米晶层组成,可在施加电压的情况下在每个电极上以2:1的比例实现总水分解为H 2和O 2在气态条件下仅为0.3V。与TiO 2电极相比,SrTiO 3气体扩散电极降低了外部电压,并抑制了涂覆在光阳极上的离聚物的逐渐分解。这些差异是由于光电流起始电位的阴极移动和O 2的法拉第效率的提高引起的。进化,分别。SrTiO 3表面和离聚物薄膜之间的界面显示出从气相提供的水吸附物中释放出O 2的快速动力学,并在紫外线照射下具有耐久性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号