当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Stereoselective assembly of 3,4-epoxypyrrolines via nucleophilic addition induced domino cyclization of 6-halo-1-oxa-4-azahexatrienes
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2019/12/26 , DOI: 10.1039/c9qo01401b Ilia A. Smetanin 1, 2, 3, 4 , Anastasiya V. Agafonova 1, 2, 3, 4 , Nikolai V. Rostovskii 1, 2, 3, 4 , Alexander F. Khlebnikov 1, 2, 3, 4 , Dmitry S. Yufit 5, 6, 7, 8 , Mikhail S. Novikov 1, 2, 3, 4
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2019/12/26 , DOI: 10.1039/c9qo01401b Ilia A. Smetanin 1, 2, 3, 4 , Anastasiya V. Agafonova 1, 2, 3, 4 , Nikolai V. Rostovskii 1, 2, 3, 4 , Alexander F. Khlebnikov 1, 2, 3, 4 , Dmitry S. Yufit 5, 6, 7, 8 , Mikhail S. Novikov 1, 2, 3, 4
Affiliation
|
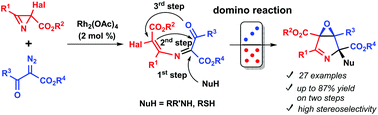
A two-step method for the preparation of 3,4-epoxypyrroline derivatives (6-oxa-3-azabicyclo[3.1.0]hex-2-enes) from 2-halo-2H-azirine-2-carboxylates, diazo keto esters, and amines has been developed. 6-Halo-1-oxa-4-azahexa-1,3,5-trienes, prepared in the first step from the azirines and diazo compounds under Rh(II) catalysis, were subjected to nucleophile-induced tandem cyclization to afford highly functionalized rac-(1R,4R,5S)-6-oxa-3-azabicyclo[3.1.0]hex-2-enes in good yields. The stereochemical outcome of the tandem cyclization induced by the secondary amines is rationalized in terms of the structural rigidity of the betaine-type precursor due to the hydrogen bonding between the ammonium group and ester group adjacent to the halogen atom. Post-modification of the 3,4-epoxypyrrolines by the Stille cross-coupling, reduction, and UV-irradiation was also demonstrated.
中文翻译:

经由亲核加成反应引起的6-卤代-1-氧杂-4-氮杂己三烯的多米诺环化反应的3,4-环氧吡咯啉立体选择性组装
分两步从2-卤代-2- H-叠氮基-2-羧酸酯,重氮酮制备3,4-环氧吡咯啉衍生物(6-oxa-3-azabicyclo [3.1.0] hex-2- enes)的方法已开发出酯和胺。第一步,在Rh(II)催化下,由叠氮基和重氮化合物制备的6-卤代-1-氧杂-4-氮杂六基-1,3,5-三烯经亲核试剂诱导的串联环化反应,以提供高度官能化的化合物rac-(1 R,4 R,5 S)-6-氧杂-3-氮杂双环[3.1.0]己-2-烯具有良好的收率。就甜菜碱型前体的结构刚性而言,仲胺诱导的串联环化的立体化学结果是合理的,这是由于邻近卤素原子的铵基团和酯基团之间存在氢键。还证明了通过Stille交叉偶联,还原和UV辐照对3,4-环氧吡咯啉进行后修饰。
更新日期:2020-02-13
中文翻译:

经由亲核加成反应引起的6-卤代-1-氧杂-4-氮杂己三烯的多米诺环化反应的3,4-环氧吡咯啉立体选择性组装
分两步从2-卤代-2- H-叠氮基-2-羧酸酯,重氮酮制备3,4-环氧吡咯啉衍生物(6-oxa-3-azabicyclo [3.1.0] hex-2- enes)的方法已开发出酯和胺。第一步,在Rh(II)催化下,由叠氮基和重氮化合物制备的6-卤代-1-氧杂-4-氮杂六基-1,3,5-三烯经亲核试剂诱导的串联环化反应,以提供高度官能化的化合物rac-(1 R,4 R,5 S)-6-氧杂-3-氮杂双环[3.1.0]己-2-烯具有良好的收率。就甜菜碱型前体的结构刚性而言,仲胺诱导的串联环化的立体化学结果是合理的,这是由于邻近卤素原子的铵基团和酯基团之间存在氢键。还证明了通过Stille交叉偶联,还原和UV辐照对3,4-环氧吡咯啉进行后修饰。



























 京公网安备 11010802027423号
京公网安备 11010802027423号