当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
[{AgL}2Mo8O26]n- complexes: a combined experimental and theoretical study.
Dalton Transactions ( IF 4 ) Pub Date : 2020-01-13 , DOI: 10.1039/c9dt04043a Anastasia V Chupina 1 , Vladimir Shayapov 2 , Alexander S Novikov 3 , Victoria V Volchek 2 , Enrico Benassi 4 , Pavel A Abramov 5 , Maxim N Sokolov 1
Dalton Transactions ( IF 4 ) Pub Date : 2020-01-13 , DOI: 10.1039/c9dt04043a Anastasia V Chupina 1 , Vladimir Shayapov 2 , Alexander S Novikov 3 , Victoria V Volchek 2 , Enrico Benassi 4 , Pavel A Abramov 5 , Maxim N Sokolov 1
Affiliation
|
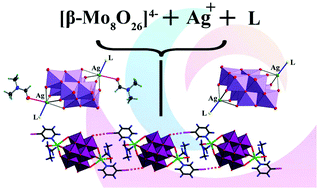
Self-assembly reactions between AgNO3, L (PPh3, PPh2Py, AsPh3, SbPh3) and [β-Mo8O26]4- in DMF led to the formation of [β-{AgL}2Mo8O26]2- anions, which were isolated as Bu4N+ salts (1-4) and characterized by XRD, IR and elemental analysis. In the crystal structures Ag+ can switch the coordination number from 5 (P, As) to 6 (Sb) by uptake of a DMF molecule. High-level QAIM analysis of the coordination sphere around Ag shows critical points even in the case of longer Ag-O distances. Changing the ligand type to a family of substituted pyridines results in novel Ag-L-POM complexes with different environments around Ag+. For 3-X-pyridine ligands (X = Cl, Br, I), complexes with additional DMF molecules [β-{AgL(DMF)}2Mo8O26]2- (5-7) have been isolated. Halogen bonding of the XO type was detected in the crystal structures of 5-7 and studied by DFT calculations, providing estimated energies from 0.9 to 3.4 kcal mol-1. Variation of substituents at the pyridine ring results in the formation of [β-{AgL}2Mo8O26]2- in the case of 2-NH2-py (8), 2-CH3-Py (9), 2,4,6-collidine (10) and 2,6-NH2-py (11). Solution behavior of 1-4 in CH3CN was studied by a hyphenated HPLC-ICP-AES technique. According to the results, the [β-{AgL}2Mo8O26]2- anions are largely dissociated in this medium. An attempt to change the [Mo8O26]4- precursor to [Mo6O19]2- (in the case of AgNO3 and PyPPh2) resulted in the crystallization of [Ag2(PyPPh2)2(DMF)4][Mo6O19] (12).
中文翻译:

[{AgL} 2Mo8O26] n-配合物:结合实验和理论研究。
AgNO3,L(PPh3,PPh2Py,AsPh3,SbPh3)和[β-Mo8O26] 4-在DMF中的自组装反应导致形成[β-{AgL} 2Mo8O26] 2-阴离子,将其分离为Bu4N +盐(1-4)并通过XRD,IR和元素分析进行表征。在晶体结构中,Ag +可以通过吸收DMF分子将配位数从5(P,As)切换为6(Sb)。对Ag周围的协调域的高级QAIM分析显示出关键点,即使在Ag-O距离较长的情况下也是如此。将配体类型更改为取代的吡啶家族,将导致新型Ag-L-POM复合物在Ag +周围具有不同的环境。对于3-X-吡啶配体(X = Cl,Br,I),已经分离了具有另外的DMF分子[β-{AgL(DMF)} 2Mo8O26] 2-(5-7)的配合物。在5-7的晶体结构中检测到XO型的卤素键,并通过DFT计算进行了研究,从而提供了从0.9到3.4 kcal mol-1的估计能量。在2-NH2-py(8),2-CH3-Py(9),2,4,6-的情况下,吡啶环上取代基的变化导致[β-{AgL} 2Mo8O26] 2-的形成可力丁(10)和2,6-NH 2 -py(11)。通过联用HPLC-ICP-AES技术研究了1-4在CH3CN中的溶液行为。根据结果,[β-{AgL} 2Mo8O26] 2-阴离子在该介质中大量解离。尝试将[Mo8O26] 4-前体更改为[Mo6O19] 2-(在AgNO3和PyPPh2的情况下)导致[Ag2(PyPPh2)2(DMF)4] [Mo6O19]结晶。在2-NH2-py(8),2-CH3-Py(9),2,4,6-的情况下,吡啶环上取代基的变化导致[β-{AgL} 2Mo8O26] 2-的形成可力丁(10)和2,6-NH 2 -py(11)。通过联用HPLC-ICP-AES技术研究了1-4在CH3CN中的溶液行为。根据结果,[β-{AgL} 2Mo8O26] 2-阴离子在该介质中大量解离。尝试将[Mo8O26] 4-前体更改为[Mo6O19] 2-(在AgNO3和PyPPh2的情况下)导致[Ag2(PyPPh2)2(DMF)4] [Mo6O19]结晶。在2-NH2-py(8),2-CH3-Py(9),2,4,6-的情况下,吡啶环上取代基的变化导致[β-{AgL} 2Mo8O26] 2-的形成可力丁(10)和2,6-NH 2 -py(11)。通过联用HPLC-ICP-AES技术研究了1-4在CH3CN中的溶液行为。根据结果,[β-{AgL} 2Mo8O26] 2-阴离子在该介质中大量解离。尝试将[Mo8O26] 4-前体更改为[Mo6O19] 2-(在AgNO3和PyPPh2的情况下)导致[Ag2(PyPPh2)2(DMF)4] [Mo6O19]结晶。
更新日期:2020-02-10
中文翻译:

[{AgL} 2Mo8O26] n-配合物:结合实验和理论研究。
AgNO3,L(PPh3,PPh2Py,AsPh3,SbPh3)和[β-Mo8O26] 4-在DMF中的自组装反应导致形成[β-{AgL} 2Mo8O26] 2-阴离子,将其分离为Bu4N +盐(1-4)并通过XRD,IR和元素分析进行表征。在晶体结构中,Ag +可以通过吸收DMF分子将配位数从5(P,As)切换为6(Sb)。对Ag周围的协调域的高级QAIM分析显示出关键点,即使在Ag-O距离较长的情况下也是如此。将配体类型更改为取代的吡啶家族,将导致新型Ag-L-POM复合物在Ag +周围具有不同的环境。对于3-X-吡啶配体(X = Cl,Br,I),已经分离了具有另外的DMF分子[β-{AgL(DMF)} 2Mo8O26] 2-(5-7)的配合物。在5-7的晶体结构中检测到XO型的卤素键,并通过DFT计算进行了研究,从而提供了从0.9到3.4 kcal mol-1的估计能量。在2-NH2-py(8),2-CH3-Py(9),2,4,6-的情况下,吡啶环上取代基的变化导致[β-{AgL} 2Mo8O26] 2-的形成可力丁(10)和2,6-NH 2 -py(11)。通过联用HPLC-ICP-AES技术研究了1-4在CH3CN中的溶液行为。根据结果,[β-{AgL} 2Mo8O26] 2-阴离子在该介质中大量解离。尝试将[Mo8O26] 4-前体更改为[Mo6O19] 2-(在AgNO3和PyPPh2的情况下)导致[Ag2(PyPPh2)2(DMF)4] [Mo6O19]结晶。在2-NH2-py(8),2-CH3-Py(9),2,4,6-的情况下,吡啶环上取代基的变化导致[β-{AgL} 2Mo8O26] 2-的形成可力丁(10)和2,6-NH 2 -py(11)。通过联用HPLC-ICP-AES技术研究了1-4在CH3CN中的溶液行为。根据结果,[β-{AgL} 2Mo8O26] 2-阴离子在该介质中大量解离。尝试将[Mo8O26] 4-前体更改为[Mo6O19] 2-(在AgNO3和PyPPh2的情况下)导致[Ag2(PyPPh2)2(DMF)4] [Mo6O19]结晶。在2-NH2-py(8),2-CH3-Py(9),2,4,6-的情况下,吡啶环上取代基的变化导致[β-{AgL} 2Mo8O26] 2-的形成可力丁(10)和2,6-NH 2 -py(11)。通过联用HPLC-ICP-AES技术研究了1-4在CH3CN中的溶液行为。根据结果,[β-{AgL} 2Mo8O26] 2-阴离子在该介质中大量解离。尝试将[Mo8O26] 4-前体更改为[Mo6O19] 2-(在AgNO3和PyPPh2的情况下)导致[Ag2(PyPPh2)2(DMF)4] [Mo6O19]结晶。



























 京公网安备 11010802027423号
京公网安备 11010802027423号