当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic diversity in acetophenone transfer hydrogenation catalyzed by ruthenium iminophosphonamide complexes.
Dalton Transactions ( IF 4 ) Pub Date : 2020-01-09 , DOI: 10.1039/c9dt04532e Alexander M Kalsin 1 , Tatyana A Peganova 1 , Iana S Sinopalnikova 2 , Ivan V Fedyanin 1 , Natalia V Belkova 1 , Eric Deydier 3 , Rinaldo Poli 4
Dalton Transactions ( IF 4 ) Pub Date : 2020-01-09 , DOI: 10.1039/c9dt04532e Alexander M Kalsin 1 , Tatyana A Peganova 1 , Iana S Sinopalnikova 2 , Ivan V Fedyanin 1 , Natalia V Belkova 1 , Eric Deydier 3 , Rinaldo Poli 4
Affiliation
|
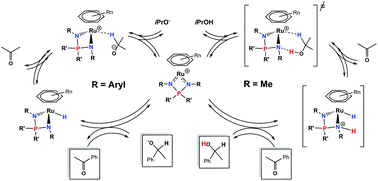
A series of arene ruthenium iminophosphonamide complexes, [(Arene)RuCl{R2P(NR')2}] (1), bearing various arenes and R,R' substituents on the NPN ligand have been investigated as precatalysts in acetophenone transfer hydrogenation in basic and base-free isopropanol. The results clearly demonstrate the presence of two distinct reaction mechanisms, which are controlled by the basicity of the N-atoms. Complexes 1 in which both R' substituents are aryl groups are only active once the neutral hydride complex [(Arene)RuH{R2P(NR')2}] (2) is generated in basic isopropanol, the latter being able to reduce a ketone via a stepwise hydride and proton transfer. On the other hand, complexes in which at least one R' group is Me readily catalyze the reaction in the absence of base. In the latter case, the results of kinetic studies and DFT calculations support an outer-sphere concerted asynchronous hydride and proton transfer assisted by the basic N-atom of the NPN ligand, which promotes catalysis via precoordination of an alcohol molecule by hydrogen bonding.
中文翻译:

钌亚氨基膦酰胺配合物催化的苯乙酮转移加氢机理的机械多样性。
研究了一系列芳烃钌亚氨基膦酰胺络合物[((Arene)RuCl {R2P(NR')2}](1),在NPN配体上带有各种芳烃和R,R'取代基,作为碱性条件下苯乙酮转移加氢的前催化剂和无碱异丙醇。结果清楚地表明存在两种不同的反应机理,这是受N原子碱性控制的。两个R'取代基均为芳基的配合物1仅在碱性异丙醇中生成中性氢化物配合物[(Arene)RuH {R2P(NR')2}](2)后才具有活性,后者能够还原酮通过逐步氢化物和质子转移。另一方面,其中至少一个R'基团为Me的络合物在不存在碱的情况下容易催化该反应。在后一种情况下,
更新日期:2020-02-10
中文翻译:

钌亚氨基膦酰胺配合物催化的苯乙酮转移加氢机理的机械多样性。
研究了一系列芳烃钌亚氨基膦酰胺络合物[((Arene)RuCl {R2P(NR')2}](1),在NPN配体上带有各种芳烃和R,R'取代基,作为碱性条件下苯乙酮转移加氢的前催化剂和无碱异丙醇。结果清楚地表明存在两种不同的反应机理,这是受N原子碱性控制的。两个R'取代基均为芳基的配合物1仅在碱性异丙醇中生成中性氢化物配合物[(Arene)RuH {R2P(NR')2}](2)后才具有活性,后者能够还原酮通过逐步氢化物和质子转移。另一方面,其中至少一个R'基团为Me的络合物在不存在碱的情况下容易催化该反应。在后一种情况下,


























 京公网安备 11010802027423号
京公网安备 11010802027423号