当前位置:
X-MOL 学术
›
Cancer Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Using circulating tumor DNA as a novel biomarker to screen and diagnose hepatocellular carcinoma: A systematic review and meta-analysis.
Cancer Medicine ( IF 4 ) Pub Date : 2019-12-26 , DOI: 10.1002/cam4.2799 Ziying Zhang 1 , Peng Chen 2 , Hui Xie 3 , Peiguo Cao 1
Cancer Medicine ( IF 4 ) Pub Date : 2019-12-26 , DOI: 10.1002/cam4.2799 Ziying Zhang 1 , Peng Chen 2 , Hui Xie 3 , Peiguo Cao 1
Affiliation
|
PURPOSE
A meta-analysis was formulated to appraise the diagnostic accuracy of circulating tumor DNA (ctDNA) in hepatocellular carcinoma (HCC).
MATERIALS AND METHODS
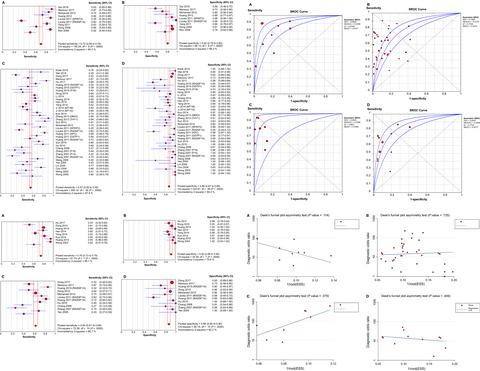
We enrolled all relevant studies published until September 2019. Four primary subgroups were investigated: the subgroup of quantitative or qualitative analysis of ctDNA, the subgroup of Ras association domain family 1 isoform A (RASSF1A) methylation in ctDNA and the subgroup of the combined alpha-fetoprotein (AFP) and ctDNA assay. We analyzed the pooled sensitivity (SEN), specificity (SPE), positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and summary receiver operating characteristic (SROC) as well as the area under the curve (AUC).
RESULTS
A total of 33 qualified articles with 4113 subjects were incorporated into our meta-analysis. The combined SEN, SPE, and DOR in quantitative studies were 0.722 (95% confidence interval (95% CI): 0.686-0.756), 0.823 (95% CI: 0.789-0.854), 18.532 (95% CI: 8.245-41.657), respectively, yielding an AUC of 0.880. For qualitative studies, the corresponding value was 0.568 (95% CI: 0.548-0.587), 0.882 (95% CI: 0.867-0.897), 10.457 (95% CI: 7.270-15.040) and 0.787, respectively. Detection of RASSF1A methylation yielded an AUC of 0.841, with a SEN of 0.644 (95% CI: 0.608-0.678) and a SPE of 0.875 (95% CI: 0.847-0.900). AFP combined with ctDNA assay achieved an AUC of 0.944, with a SEN of 0.760 (95% CI: 0.728-00.790) and a SPE of 0.920 (95% CI: 0.893-00.942).
CONCLUSION
Circulating tumor DNA displays a promising diagnostic potential in HCC. However, it is not independently sufficient and can serve as an assistant tool combined with AFP for HCC screening and detection.
中文翻译:

使用循环肿瘤DNA作为筛选和诊断肝细胞癌的新型生物标志物:系统评价和荟萃分析。
目的制定一项荟萃分析,以评估循环肿瘤DNA(ctDNA)在肝细胞癌(HCC)中的诊断准确性。材料和方法我们纳入了所有直至2019年9月发表的相关研究。研究了四个主要亚组:ctDNA的定量或定性分析亚组,ctDNA中Ras缔合域家族1亚型A(RASSF1A)甲基化的亚组和ctDNA的亚组。结合甲胎蛋白(AFP)和ctDNA检测。我们分析了合并的灵敏度(SEN),特异性(SPE),正似然比(PLR),负似然比(NLR),诊断比值比(DOR)和摘要接收器操作特征(SROC)以及分析区域下的面积曲线(AUC)。结果我们的荟萃分析共纳入33篇合格文章,涉及4113个主题。定量研究中的SEN,SPE和DOR组合为0.722(95%置信区间(95%CI):0.686-0.756),0.823(95%CI:0.789-0.854),18.532(95%CI:8.245-41.657)分别得出0.880的AUC。对于定性研究,相应的值分别为0.568(95%CI:0.548-0.587),0.882(95%CI:0.867-0.897),10.457(95%CI:7.270-15.040)和0.787。RASSF1A甲基化的检测产生0.841的AUC,SEN为0.644(95%CI:0.608-0.678)和SPE为0.875(95%CI:0.847-0.900)。AFP与ctDNA分析相结合,AUC为0.944,SEN为0.760(95%CI:0.728-00.790),SPE为0.920(95%CI:0.893-00.942)。结论循环中的肿瘤DNA在HCC中显示出有希望的诊断潜力。然而,
更新日期:2019-12-27
中文翻译:

使用循环肿瘤DNA作为筛选和诊断肝细胞癌的新型生物标志物:系统评价和荟萃分析。
目的制定一项荟萃分析,以评估循环肿瘤DNA(ctDNA)在肝细胞癌(HCC)中的诊断准确性。材料和方法我们纳入了所有直至2019年9月发表的相关研究。研究了四个主要亚组:ctDNA的定量或定性分析亚组,ctDNA中Ras缔合域家族1亚型A(RASSF1A)甲基化的亚组和ctDNA的亚组。结合甲胎蛋白(AFP)和ctDNA检测。我们分析了合并的灵敏度(SEN),特异性(SPE),正似然比(PLR),负似然比(NLR),诊断比值比(DOR)和摘要接收器操作特征(SROC)以及分析区域下的面积曲线(AUC)。结果我们的荟萃分析共纳入33篇合格文章,涉及4113个主题。定量研究中的SEN,SPE和DOR组合为0.722(95%置信区间(95%CI):0.686-0.756),0.823(95%CI:0.789-0.854),18.532(95%CI:8.245-41.657)分别得出0.880的AUC。对于定性研究,相应的值分别为0.568(95%CI:0.548-0.587),0.882(95%CI:0.867-0.897),10.457(95%CI:7.270-15.040)和0.787。RASSF1A甲基化的检测产生0.841的AUC,SEN为0.644(95%CI:0.608-0.678)和SPE为0.875(95%CI:0.847-0.900)。AFP与ctDNA分析相结合,AUC为0.944,SEN为0.760(95%CI:0.728-00.790),SPE为0.920(95%CI:0.893-00.942)。结论循环中的肿瘤DNA在HCC中显示出有希望的诊断潜力。然而,


























 京公网安备 11010802027423号
京公网安备 11010802027423号