当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic Investigation of Haloacetic Acid Reduction Using Carbon-Ti4O7 Composite Reactive Electrochemical Membranes.
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2020-01-13 , DOI: 10.1021/acs.est.9b06744 Soroush Almassi 1 , Pamela Rose V Samonte 2 , Zhao Li 3 , Wenqing Xu 3 , Brian P Chaplin 1
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2020-01-13 , DOI: 10.1021/acs.est.9b06744 Soroush Almassi 1 , Pamela Rose V Samonte 2 , Zhao Li 3 , Wenqing Xu 3 , Brian P Chaplin 1
Affiliation
|
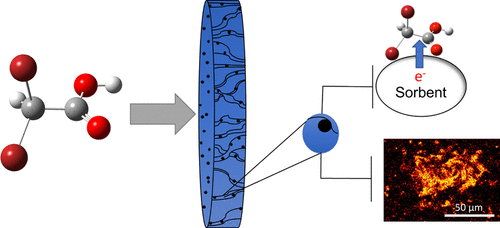
Carbon-Ti4O7 composite reactive electrochemical membranes (REMs) were studied for adsorption and electrochemical reduction of haloacetic acids (HAAs). Powder activated carbon (PAC) or multiwalled carbon nanotubes (MWCNTs) were used in these composites. Results from flow-through adsorption experiments with dibromoacetic acid (DBAA) as a model HAA were interpreted with a transport model. It was estimated that ∼46% of C in the MWCNT-REM and ∼10% of C in the PAC-REM participated in adsorption reactions. Electrochemical reduction of 1 mg L-1 DBAA in 10 mM KH2PO4/K2HPO4 at -1.5 V/SHE (hydraulic residence time, ∼11 s) resulted in 73, 94, and 96% DBAA reduction for Ti4O7, PAC-Ti4O7, and MWCNT-Ti4O7 REMs, respectively. The reactive-transport model yielded kobs values between 9.16 and 33.3 min-1, which were 2 to 4 orders of magnitude higher than previously reported. PAC-Ti4O7 REM was tested with tap water spiked with 0.11 mg L-1 of nine different HAAs in a similar reduction experiment. The results indicated that all HAAs were reduced to <20 μg L-1. Moreover, the total combined concentration of five regulated HAAs was lower than the regulatory limit (60 μg L-1). Density functional theory simulations suggest that a direct electron transfer reaction was the probable rate-determining step for HAA reduction.
中文翻译:

碳-Ti4O7复合活性电化学膜还原卤代乙酸的机理研究。
研究了碳-Ti4O7复合反应性电化学膜(REMs)对卤代乙酸(HAAs)的吸附和电化学还原。在这些复合材料中使用了粉末活性炭(PAC)或多壁碳纳米管(MWCNT)。用运输模型解释了使用二溴乙酸(DBAA)作为HAA模型的流通式吸附实验的结果。据估计,MWCNT-REM中约46%的C和PAC-REM中约10%的C参与了吸附反应。在-1.5 V / SHE(水停留时间,约11 s)下,在10 mM KH2PO4 / K2HPO4中电化学还原1 mg L-1 DBAA导致Ti4O7,PAC-Ti4O7和MWCNT的DBAA降低73%,94%和96% -Ti4O7 REM。反应运输模型产生的kobs值介于9.16和33.3 min-1之间,比以前报道的高2到4个数量级。在类似的还原实验中,用0.11 mg L-1的9种不同HAAs加标的自来水对PAC-Ti4O7 REM进行了测试。结果表明,所有HAA均降至<20μgL-1。此外,五种被调节的HAA的总结合浓度低于调节极限(60μgL-1)。密度泛函理论模拟表明,直接电子转移反应是降低HAA的速率决定步骤。
更新日期:2020-01-13
中文翻译:

碳-Ti4O7复合活性电化学膜还原卤代乙酸的机理研究。
研究了碳-Ti4O7复合反应性电化学膜(REMs)对卤代乙酸(HAAs)的吸附和电化学还原。在这些复合材料中使用了粉末活性炭(PAC)或多壁碳纳米管(MWCNT)。用运输模型解释了使用二溴乙酸(DBAA)作为HAA模型的流通式吸附实验的结果。据估计,MWCNT-REM中约46%的C和PAC-REM中约10%的C参与了吸附反应。在-1.5 V / SHE(水停留时间,约11 s)下,在10 mM KH2PO4 / K2HPO4中电化学还原1 mg L-1 DBAA导致Ti4O7,PAC-Ti4O7和MWCNT的DBAA降低73%,94%和96% -Ti4O7 REM。反应运输模型产生的kobs值介于9.16和33.3 min-1之间,比以前报道的高2到4个数量级。在类似的还原实验中,用0.11 mg L-1的9种不同HAAs加标的自来水对PAC-Ti4O7 REM进行了测试。结果表明,所有HAA均降至<20μgL-1。此外,五种被调节的HAA的总结合浓度低于调节极限(60μgL-1)。密度泛函理论模拟表明,直接电子转移反应是降低HAA的速率决定步骤。


























 京公网安备 11010802027423号
京公网安备 11010802027423号